Empagliflozin inhibits increased Na influx in atrial cardiomyocytes of patients with HFpEF
- PMID: 38728438
- PMCID: PMC11288740
- DOI: 10.1093/cvr/cvae095
Empagliflozin inhibits increased Na influx in atrial cardiomyocytes of patients with HFpEF
Abstract
Aims: Heart failure with preserved ejection fraction (HFpEF) causes substantial morbidity and mortality. Importantly, atrial remodelling and atrial fibrillation are frequently observed in HFpEF. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have recently been shown to improve clinical outcomes in HFpEF, and post-hoc analyses suggest atrial anti-arrhythmic effects. We tested if isolated human atrial cardiomyocytes from patients with HFpEF exhibit an increased Na influx, which is known to cause atrial arrhythmias, and if that is responsive to treatment with the SGTL2i empagliflozin.
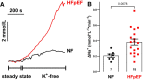
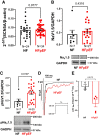
Methods and results: Cardiomyocytes were isolated from atrial biopsies of 124 patients (82 with HFpEF) undergoing elective cardiac surgery. Na influx was measured with the Na-dye Asante Natrium Green-2 AM (ANG-2). Compared to patients without heart failure (NF), Na influx was doubled in HFpEF patients (NF vs. HFpEF: 0.21 ± 0.02 vs. 0.38 ± 0.04 mmol/L/min (N = 7 vs. 18); P = 0.0078). Moreover, late INa (measured via whole-cell patch clamp) was significantly increased in HFpEF compared to NF. Western blot and HDAC4 pulldown assay indicated a significant increase in CaMKII expression, CaMKII autophosphorylation, CaMKII activity, and CaMKII-dependent NaV1.5 phosphorylation in HFpEF compared to NF, whereas NaV1.5 protein and mRNA abundance remained unchanged. Consistently, increased Na influx was significantly reduced by treatment not only with the CaMKII inhibitor autocamtide-2-related inhibitory peptide (AIP), late INa inhibitor tetrodotoxin (TTX) but also with sodium/hydrogen exchanger 1 (NHE1) inhibitor cariporide. Importantly, empagliflozin abolished both increased Na influx and late INa in HFpEF. Multivariate linear regression analysis, adjusting for important clinical confounders, revealed HFpEF to be an independent predictor for changes in Na handling in atrial cardiomyocytes.
Conclusion: We show for the first time increased Na influx in human atrial cardiomyocytes from HFpEF patients, partly due to increased late INa and enhanced NHE1-mediated Na influx. Empagliflozin inhibits Na influx and late INa, which could contribute to anti-arrhythmic effects in patients with HFpEF.
Keywords: Atrial remodelling; Empagliflozin; HFpEF; Heart failure; SGLT2i; Sodium.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: L.S.M. and S.W. receive compensation for talks for Boehringer Ingelheim, the company that sells empagliflozin. The other authors declare to have no conflict of interest associated with this manuscript.
Figures




References
-
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D-J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca H-P, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde M-F, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. - PubMed
-
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. - PubMed
-
- Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol 1998;32:695–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

