Neurotransmitter classification from electron microscopy images at synaptic sites in Drosophila melanogaster
- PMID: 38729112
- PMCID: PMC11106717
- DOI: 10.1016/j.cell.2024.03.016
Neurotransmitter classification from electron microscopy images at synaptic sites in Drosophila melanogaster
Abstract
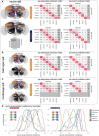
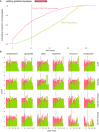
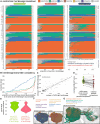
High-resolution electron microscopy of nervous systems has enabled the reconstruction of synaptic connectomes. However, we do not know the synaptic sign for each connection (i.e., whether a connection is excitatory or inhibitory), which is implied by the released transmitter. We demonstrate that artificial neural networks can predict transmitter types for presynapses from electron micrographs: a network trained to predict six transmitters (acetylcholine, glutamate, GABA, serotonin, dopamine, octopamine) achieves an accuracy of 87% for individual synapses, 94% for neurons, and 91% for known cell types across a D. melanogaster whole brain. We visualize the ultrastructural features used for prediction, discovering subtle but significant differences between transmitter phenotypes. We also analyze transmitter distributions across the brain and find that neurons that develop together largely express only one fast-acting transmitter (acetylcholine, glutamate, or GABA). We hope that our publicly available predictions act as an accelerant for neuroscientific hypothesis generation for the fly.
Keywords: neuroscience, machine learning, electron microscopy, Drosophila melanogaster, neurotransmitter, explainable AI.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures










References
-
- Ohyama T., Schneider-Mizell C.M., Fetter R.D., Aleman J.V., Franconville R., Rivera-Alba M., Mensh B.D., Branson K.M., Simpson J.H., Truman J.W., et al. A multilevel multimodal circuit enhances action selection in drosophila. Nature. 2015;520:633–639. - PubMed
-
- Takemura S.-Y., Hayworth K.J., Huang G.B., Januszewski M., Lu Z., Marin E.C., Preibisch S., Shan Xu C., Bogovic J., Champion A.S., et al. A connectome of the male drosophila ventral nerve cord. bioRxiv. 2023 doi: 10.1101/2023.06.05.543757. Preprint at. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

