A latent pool of neurons silenced by sensory-evoked inhibition can be recruited to enhance perception
- PMID: 38729150
- PMCID: PMC7616379
- DOI: 10.1016/j.neuron.2024.04.015
A latent pool of neurons silenced by sensory-evoked inhibition can be recruited to enhance perception
Abstract
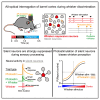
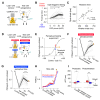
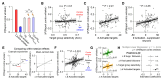
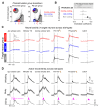
To investigate which activity patterns in sensory cortex are relevant for perceptual decision-making, we combined two-photon calcium imaging and targeted two-photon optogenetics to interrogate barrel cortex activity during perceptual discrimination. We trained mice to discriminate bilateral whisker deflections and report decisions by licking left or right. Two-photon calcium imaging revealed sparse coding of contralateral and ipsilateral whisker input in layer 2/3, with most neurons remaining silent during the task. Activating pyramidal neurons using two-photon holographic photostimulation evoked a perceptual bias that scaled with the number of neurons photostimulated. This effect was dominated by optogenetic activation of non-coding neurons, which did not show sensory or motor-related activity during task performance. Photostimulation also revealed potent recruitment of cortical inhibition during sensory processing, which strongly and preferentially suppressed non-coding neurons. Our results suggest that a pool of non-coding neurons, selectively suppressed by network inhibition during sensory processing, can be recruited to enhance perception.
Keywords: all-optical interrogation; behavior; cortex; optogenetics; sensory processing; sparse coding; two-photon imaging.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

