Long-acting intramuscular injections of ELQ-331, an antimalarial agent
- PMID: 38729224
- PMCID: PMC11160314
- DOI: 10.1016/j.ejps.2024.106795
Long-acting intramuscular injections of ELQ-331, an antimalarial agent
Abstract
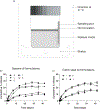
The overarching premise of this investigation is that injectable, long-acting antimalarial medication would encourage adherence to a dosage regimen for populations at risk of contracting the disease. To advance support for this goal, we have developed oil-based formulations of ELQ-331 (a prodrug of ELQ-300) that perform as long-acting, injectable chemoprophylactics with drug loading as high as 160 mg/ml of ELQ-331. In a pharmacokinetic study performed with rats, a single intramuscular injection of 12.14 mg/kg maintained higher plasma levels than the previously established minimum fully protective plasma concentration (33.25 ng/ml) of ELQ-300 for more than 4 weeks. The formulations were well tolerated by the rats and the tested dose produced no adverse reactions. We believe that by extending the length of time between subsequent injections, these injectable oil-based solutions of ELQ-331 can offer a more accessible, low-cost option for long-acting disease prevention and reduced transmission in malaria-endemic regions and may also be of use to travelers.
Keywords: Antimalarial; ELQ; Long-acting injectable chemoprophylactic (LAI-C); Oil-based intramuscular injection.
Copyright © 2024. Published by Elsevier B.V.
Figures

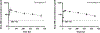
Similar articles
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
-
Paliperidone palmitate for schizophrenia.Cochrane Database Syst Rev. 2012 Jun 13;2012(6):CD008296. doi: 10.1002/14651858.CD008296.pub2. Cochrane Database Syst Rev. 2012. PMID: 22696377 Free PMC article.
-
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972056 Free PMC article.
-
Primaquine for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008152. doi: 10.1002/14651858.CD008152.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. PMID: 22972117 Updated.
References
-
- Avital-Shmilovici M, Liu X, Shaler T, Lowenthal A, Bourbon P, Snider J, Tambo-Ong A, Repellin C, Yniguez K, Sambucetti L, Madrid PB, Collins N.,2022, Mega-High-Throughput Screening Platform for the Discovery of Biologically Relevant Sequence-Defined Non-Natural Polymers. ACS Cent Sci. 8(1):86–101. doi: 10.1021/acscentsci.1c01041. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

