A reliable deep-learning-based method for alveolar bone quantification using a murine model of periodontitis and micro-computed tomography imaging
- PMID: 38729290
- PMCID: PMC11288397
- DOI: 10.1016/j.jdent.2024.105057
A reliable deep-learning-based method for alveolar bone quantification using a murine model of periodontitis and micro-computed tomography imaging
Abstract
Objectives: This study focuses on artificial intelligence (AI)-assisted analysis of alveolar bone for periodontitis in a mouse model with the aim to create an automatic deep-learning segmentation model that enables researchers to easily examine alveolar bone from micro-computed tomography (µCT) data without needing prior machine learning knowledge.
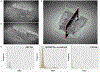
Methods: Ligature-induced experimental periodontitis was produced by placing a small-diameter silk sling ligature around the left maxillary second molar. At 4, 7, 9, or 14 days, the maxillary bone was harvested and processed with a µCT scanner (µCT-45, Scanco). Using Dragonfly (v2021.3), we developed a 3D deep learning model based on the U-Net AI deep learning engine for segmenting materials in complex images to measure alveolar bone volume (BV) and bone mineral density (BMD) while excluding the teeth from the measurements.
Results: This model generates 3D segmentation output for a selected region of interest with over 98 % accuracy on different formats of µCT data. BV on the ligature side gradually decreased from 0.87 mm3 to 0.50 mm3 on day 9 and then increased to 0.63 mm3 on day 14. The ligature side lost 4.6 % of BMD on day 4, 9.6 % on day 7, 17.7 % on day 9, and 21.1 % on day 14.
Conclusions: This study developed an AI model that can be downloaded and easily applied, allowing researchers to assess metrics including BV, BMD, and trabecular bone thickness, while excluding teeth from the measurements of mouse alveolar bone.
Clinical significance: This work offers an innovative, user-friendly automatic segmentation model that is fast, accurate, and reliable, demonstrating new potential uses of artificial intelligence (AI) in dentistry with great potential in diagnosing, treating, and prognosis of oral diseases.
Keywords: Deep learning; Micro-CT; Periodontitis.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors have no competing interests to declare.
Figures




References
-
- Kassebaum NJ, Smith AGC, Bernabé E, Fleming TD, Reynolds AE, Vos T, Murray CJL, Marcenes W, Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors, Journal of dental research 96(4) (2017) 380–387. - PMC - PubMed
-
- Kinane DF, Stathopoulou PG, Papapanou PN, Periodontal diseases, Nature reviews. Disease primers 3 (2017) 17038. - PubMed
-
- Caton JG, Armitage G, Berglundh T, Chapple IL, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS, A new classification scheme for periodontal and peri‐implant diseases and conditions–Introduction and key changes from the 1999 classification, Journal of periodontology 89 (2018) S1–S8. - PubMed
-
- Baker PJ, Evans RT, Roopenian D.C.J.A.o.o.b., Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice, 39(12) (1994) 1035–1040. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

