Mesenchymal stem cell secretome for regenerative medicine: Where do we stand?
- PMID: 38729561
- PMCID: PMC11976416
- DOI: 10.1016/j.jare.2024.05.004
Mesenchymal stem cell secretome for regenerative medicine: Where do we stand?
Abstract
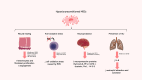
Background: Mesenchymal stem cell (MSC)-based therapies have yielded beneficial effects in a broad range of preclinical models and clinical trials for human diseases. In the context of MSC transplantation, it is widely recognized that the main mechanism for the regenerative potential of MSCs is not their differentiation, with in vivo data revealing transient and low engraftment rates. Instead, MSCs therapeutic effects are mainly attributed to its secretome, i.e., paracrine factors secreted by these cells, further offering a more attractive and innovative approach due to the effectiveness and safety of a cell-free product.
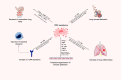
Aim of review: In this review, we will discuss the potential benefits of MSC-derived secretome in regenerative medicine with particular focus on respiratory, hepatic, and neurological diseases. Both free and vesicular factors of MSC secretome will be detailed. We will also address novel potential strategies capable of improving their healing potential, namely by delivering important regenerative molecules according to specific diseases and tissue needs, as well as non-clinical and clinical studies that allow us to dissect their mechanisms of action.
Key scientific concepts of review: MSC-derived secretome includes both soluble and non-soluble factors, organized in extracellular vesicles (EVs). Importantly, besides depending on the cell origin, the characteristics and therapeutic potential of MSC secretome is deeply influenced by external stimuli, highlighting the possibility of optimizing their characteristics through preconditioning approaches. Nevertheless, the clarity around their mechanisms of action remains ambiguous, whereas the need for standardized procedures for the successful translation of those products to the clinics urges.
Keywords: Extracellular vesicles; Mesenchymal stem cells; Preconditioning strategies; Regenerative medicine; Secretome.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Farfán N., Carril J., Redel M., Zamorano M., Araya M., Monzón E., et al. Intranasal administration of mesenchymal stem cell secretome reduces hippocampal oxidative stress, neuroinflammation and cell death, improving the behavioral outcome following perinatal asphyxia. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21207800. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

