Pretreatment with IL-15 and IL-18 rescues natural killer cells from granzyme B-mediated apoptosis after cryopreservation
- PMID: 38729924
- PMCID: PMC11087472
- DOI: 10.1038/s41467-024-47574-0
Pretreatment with IL-15 and IL-18 rescues natural killer cells from granzyme B-mediated apoptosis after cryopreservation
Abstract
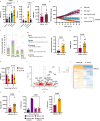
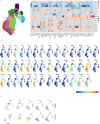
Human natural killer (NK) cell-based therapies are under assessment for treating various cancers, but cryopreservation reduces both the recovery and function of NK cells, thereby limiting their therapeutic feasibility. Using cryopreservation protocols optimized for T cells, here we find that ~75% of NK cells die within 24 h post-thaw, with the remaining cells displaying reduced cytotoxicity. Using CRISPR-Cas9 gene editing and confocal microscopy, we find that cryopreserved NK cells largely die via apoptosis initiated by leakage of granzyme B from cytotoxic vesicles. Pretreatment of NK cells with a combination of Interleukins-15 (IL-15) and IL-18 prior to cryopreservation improves NK cell recovery to ~90-100% and enables equal tumour control in a xenograft model of disseminated Raji cell lymphoma compared to non-cryopreserved NK cells. The mechanism of IL-15 and IL-18-induced protection incorporates two mechanisms: a transient reduction in intracellular granzyme B levels via degranulation, and the induction of antiapoptotic genes.
© 2024. The Author(s).
Conflict of interest statement
A.B. and N.C.S. have submitted a Patent application covering aspects of this work. C.H.J. has received grant support from Novartis, and has patents related to CAR-Therapy with royalties paid from Novartis to the University of Pennsylvania. C.H.J. is also a scientific co-founder and holds equity in BlueWhale Bio, Capstan Therapeutics and Tmunity Therapeutics. He serves on the board of AC Immune and is a scientific advisor Alaunos, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Cellcarta, Celldex, Danaher, Decheng, ImmuneSensor, Poseida, Verismo, Viracta, and WIRB-Copernicus group. L.L.L. is an advisor to Catamaran, Cullinan Oncology, Dragonfly, DrenBio, Edity, GV20, IMIDomics, InnDura Therapeutics, Innovent, Nkarta, oNKo-innate, Obsidian Therapeutics, and SBI Biotech. N.C.S. holds equity in Tmunity Therapeutics and BlueWhale Bio. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

