Deciphering complexity in Pd-catalyzed cross-couplings
- PMID: 38729925
- PMCID: PMC11087562
- DOI: 10.1038/s41467-024-47939-5
Deciphering complexity in Pd-catalyzed cross-couplings
Abstract
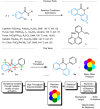
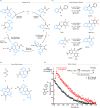
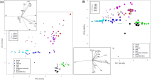
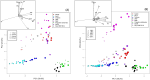
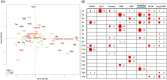
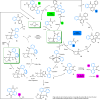
Understanding complex reaction systems is critical in chemistry. While synthetic methods for selective formation of products are sought after, oftentimes it is the full reaction signature, i.e., complete profile of products/side-products, that informs mechanistic rationale and accelerates discovery chemistry. Here, we report a methodology using high-throughput experimentation and multivariate data analysis to examine the full signature of one of the most complicated chemical reactions catalyzed by palladium known in the chemical literature. A model Pd-catalyzed reaction was selected involving functionalization of 2-bromo-N-phenylbenzamide and multiple bond activation pathways. Principal component analysis, correspondence analysis and heatmaps with hierarchical clustering reveal the factors contributing to the variance in product distributions and show associations between solvents and reaction products. Using robust data from experiments performed with eight solvents, for four different reaction times at five different temperatures, we correlate side-products to a major dominant N-phenyl phenanthridinone product, and many other side products.
© 2024. The Author(s).
Conflict of interest statement
I.J.S.F. declares a potential competing interest associated with this research. All other authors do not have competing interests. Working in partnership with Chemspeed Technologies, ISYNTH equipment was embedded within the Chemistry laboratories in York in 2012. A contract with the University of York was put in place for the Chemspeed Technologies UK to be based in York from 2012–19. All research conducted on the equipment was free from direct involvement with the company. The research did not require prior approval for publication of this research. The company checked the acknowledgements section prior to submission of the manuscript for peer review.
Figures



References
-
- Broadbelt LJ, Pfaendtner J. Lexicography of kinetic modeling of complex reaction networks. AIChE J. 2005;51:2112–2121. doi: 10.1002/aic.10599. - DOI
-
- Scott NWJ, et al. The ubiquitous cross-coupling catalyst system ‘Pd(OAc)2’/2PPh3 forms a unique dinuclear PdI complex: an important entry point into catalytically competent cyclic Pd3 clusters. Chem. Sci. 2019;10:7898–7906. doi: 10.1039/C9SC01847F. - DOI
-
- Amatore C, Carre E, Jutand A, M’Barki MA. Rates and mechanism of the formation of zerovalent palladium complexes from mixtures of Pd(OAc)2 and tertiary phosphines and their reactivity in oxidative additions. Organometallics. 1995;14:1818–1826. doi: 10.1021/om00004a039. - DOI
LinkOut - more resources
Full Text Sources

