Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2
- PMID: 38730068
- PMCID: PMC11087556
- DOI: 10.1038/s41598-024-61662-7
Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2
Abstract
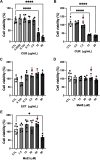
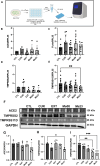
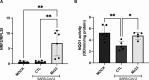
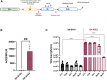
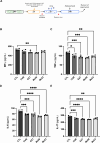
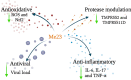
COVID-19, caused by SARS-CoV-2, affects neuronal cells, causing several symptoms such as memory loss, anosmia and brain inflammation. Curcuminoids (Me08 e Me23) and curcumin (CUR) are derived from Curcuma Longa extract (EXT). Many therapeutic actions have been linked to these compounds, including antiviral action. Given the severe implications of COVID-19, especially within the central nervous system, our study aims to shed light on the therapeutic potential of curcuminoids against SARS-CoV-2 infection, particularly in neuronal cells. Here, we investigated the effects of CUR, EXT, Me08 and Me23 in human neuroblastoma SH-SY5Y. We observed that Me23 significantly decreased the expression of plasma membrane-associated transmembrane protease serine 2 (TMPRSS2) and TMPRSS11D, consequently mitigating the elevated ROS levels induced by SARS-CoV-2. Furthermore, Me23 exhibited antioxidative properties by increasing NRF2 gene expression and restoring NQO1 activity following SARS-CoV-2 infection. Both Me08 and Me23 effectively reduced SARS-CoV-2 replication in SH-SY5Y cells overexpressing ACE2 (SH-ACE2). Additionally, all of these compounds demonstrated the ability to decrease proinflammatory cytokines such as IL-6, TNF-α, and IL-17, while Me08 specifically reduced INF-γ levels. Our findings suggest that curcuminoid Me23 could serve as a potential agent for mitigating the impact of COVID-19, particularly within the context of central nervous system involvement.
Keywords: COVID19; Curcumin; Neuroinflammation; Neuronal cell; Oxidative stress.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- 2021/2023/Fundação de Apoio à Pesquisa, Faculdade de Ciências Médicas da Santa Casa de São Paulo
- 2021/2023/Fundação de Apoio à Pesquisa, Faculdade de Ciências Médicas da Santa Casa de São Paulo
- 2020/13480-4/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2016/20796-2/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2019/10922-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

