CYP3A4 inhibitors may influence the quantification of [123I]I-FP-CIT SPECT scans
- PMID: 38730086
- PMCID: PMC11369057
- DOI: 10.1007/s00259-024-06748-0
CYP3A4 inhibitors may influence the quantification of [123I]I-FP-CIT SPECT scans
Abstract
Purpose: [123I]I-FP-CIT SPECT is an imaging tool to support the diagnosis of parkinsonian syndromes characterized by nigrostriatal dopaminergic degeneration. After intravenous injection, [123I]I-FP-CIT is metabolized for a small part by the enzyme CYP3A4, leading to the formation of [123I]I-nor-β-CIT. [123I]I-nor-β-CIT passes the blood-brain barrier and has a very high affinity for the serotonin transporter (SERT). The SERT is expressed in the striatum and cortical areas. So, at least theoretical, the use of frequently used CYP3A4 inhibitors (like amiodarone) may influence the specific to non-specific striatal [123I]I-FP-CIT ratio. Here we tested this novel hypothesis.
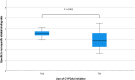
Methods: Using a retrospective design, we determined the specific to non-specific striatal [123I]I-FP-CIT ratio (using BRASS software) in 6 subjects that were using an CYP3A4 inhibitor and 18 matched controls. Only subjects were included with a normal rated [123I]I-FP-CIT SPECT scan, and all participants were scanned on the same brain-dedicated SPECT system.
Results: The specific to non-specific (assessed in the occipital cortex) striatal [123I]I-FP-CIT binding ratio was significantly higher in CYP3A4 users than in the control group (3.52 ± 0.33 vs. 2.90 ± 0.78, p < 0.001).
Conclusion: Our preliminary data suggest that the use of CYP3A4 inhibitors may influence striatal [123I]I-FP-CIT binding ratios. This information, when reproduced in larger studies, may be relevant for studies in which quantification of [123I]I-FP-CIT SPECT imaging is used for diagnostic or research purposes.
Keywords: CYP3A4 inhibitors; DaTSCAN; Dopamine transporter imaging; Drug interactions; [123I]I-FP-CIT.
© 2024. The Author(s).
Conflict of interest statement
Author ZHS is an employee of GE Healthcare. Author JB is a consultant of GE Healthcare and received research funding from GE Healthcare (all payments to Amsterdam UMC).
Figures


References
-
- Chahid Y, Sheikh ZH, Mitropoulos M, Booij J. A systematic review of the potential effects of medications and drugs of abuse on dopamine transporter imaging using [(123)I]I-FP-CIT SPECT in routine practice. Eur J Nucl Med Mol Imaging. 2023;50:1974–87. 10.1007/s00259-023-06171-x. 10.1007/s00259-023-06171-x - DOI - PMC - PubMed
-
- Chaly T, Dhawan V, Kazumata K, Antonini A, Margouleff C, Dahl JR, et al. Radiosynthesis of [18F] N-3-fluoropropyl-2-beta-carbomethoxy-3-beta-(4-iodophenyl) nortropane and the first human study with positron emission tomography. Nucl Med Biol. 1996;23:999–1004. 10.1016/s0969-8051(96)00155-2. 10.1016/s0969-8051(96)00155-2 - DOI - PubMed
-
- Bergstrom KA, Halldin C, Lundkvist C, Swahn CG, Akerman KK, Kuikka JT, et al. Characterization of C-11 or I-123 labelled beta-CIT-FP and beta-CIT-FE metabolism measured in monkey and human plasma. Identification of two labelled metabolites with HPLC. Hum Psychopharm Clin. 1996;11:483–90. doi:Doi 10.1002/(Sici)1099 – 1077(199611)11:6 < 483::Aid-Hup818 > 3.0.Co;2–9.10.1002/(SICI)1099-1077(199611)11:6<483::AID-HUP818>3.0.CO;2-9 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

