Contribution of extracellular vesicles for the pathogenesis of retinal diseases: shedding light on blood-retinal barrier dysfunction
- PMID: 38730462
- PMCID: PMC11088087
- DOI: 10.1186/s12929-024-01036-3
Contribution of extracellular vesicles for the pathogenesis of retinal diseases: shedding light on blood-retinal barrier dysfunction
Abstract
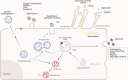
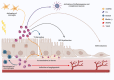
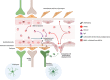
Retinal degenerative diseases, including diabetic retinopathy (DR) and age-related macular degeneration (AMD), loom as threats to vision, causing detrimental effects on the structure and function of the retina. Central to understanding these diseases, is the compromised state of the blood-retinal barrier (BRB), an effective barrier that regulates the influx of immune and inflammatory components. Whether BRB breakdown initiates retinal distress, or is a consequence of disease progression, remains enigmatic. Nevertheless, it is an indication of retinal dysfunction and potential vision loss.The intricate intercellular dialogues among retinal cell populations remain unintelligible in the complex retinal milieu, under conditions of inflammation and oxidative stress. The retina, a specialized neural tissue, sustains a ceaseless demand for oxygen and nutrients from two vascular networks. The BRB orchestrates the exchange of molecules and fluids within this specialized region, comprising the inner BRB (iBRB) and the outer BRB (oBRB). Extracellular vesicles (EVs) are small membranous structures, and act as messengers facilitating intercellular communication in this milieu.EVs, both from retinal and peripheral immune cells, increase complexity to BRB dysfunction in DR and AMD. Laden with bioactive cargoes, these EVs can modulate the retinal microenvironment, influencing disease progression. Our review delves into the multifaceted role of EVs in retinal degenerative diseases, elucidating the molecular crosstalk they orchestrate, and their microRNA (miRNA) content. By shedding light on these nanoscale messengers, from their biogenesis, release, to interaction and uptake by target cells, we aim to deepen the comprehension of BRB dysfunction and explore their therapeutic potential, therefore increasing our understanding of DR and AMD pathophysiology.
Keywords: Age-related macular degeneration; Blood-retinal barrier; Diabetic retinopathy; Extracellular vesicles; Retinal degenerative diseases; miRNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction.Biomolecules. 2020 Jul 29;10(8):1119. doi: 10.3390/biom10081119. Biomolecules. 2020. PMID: 32751132 Free PMC article. Review.
-
The blood-retina barrier in health and disease.FEBS J. 2023 Feb;290(4):878-891. doi: 10.1111/febs.16330. Epub 2021 Dec 28. FEBS J. 2023. PMID: 34923749 Review.
-
Blood-retinal barrier.Eur J Ophthalmol. 2011;21 Suppl 6:S3-9. doi: 10.5301/EJO.2010.6049. Eur J Ophthalmol. 2011. PMID: 23264323 Review.
-
The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration.Cells. 2021 Jan 4;10(1):64. doi: 10.3390/cells10010064. Cells. 2021. PMID: 33406612 Free PMC article. Review.
-
Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management.Prog Retin Eye Res. 2008 Nov;27(6):622-47. doi: 10.1016/j.preteyeres.2008.09.003. Epub 2008 Oct 4. Prog Retin Eye Res. 2008. PMID: 18940262 Review.
Cited by
-
Pro-Inflammatory Characteristics of Extracellular Vesicles in the Vitreous of Type 2 Diabetic Patients.Biomedicines. 2024 Sep 10;12(9):2053. doi: 10.3390/biomedicines12092053. Biomedicines. 2024. PMID: 39335566 Free PMC article.
-
Exosomal ncRNAs in reproductive cancers†.Biol Reprod. 2025 Feb 14;112(2):225-244. doi: 10.1093/biolre/ioae170. Biol Reprod. 2025. PMID: 39561105 Free PMC article. Review.
-
In Vitro Models of Diabetes: Focus on Diabetic Retinopathy.Cells. 2024 Nov 11;13(22):1864. doi: 10.3390/cells13221864. Cells. 2024. PMID: 39594613 Free PMC article. Review.
-
Recent advances in engineered exosome-based therapies for ocular vascular disease.J Nanobiotechnology. 2025 Jul 19;23(1):526. doi: 10.1186/s12951-025-03589-3. J Nanobiotechnology. 2025. PMID: 40684186 Free PMC article. Review.
-
Spatial characterization of RPE structure and lipids in the PEX1-p.Gly844Asp mouse model for Zellweger spectrum disorder.J Lipid Res. 2025 Apr;66(4):100771. doi: 10.1016/j.jlr.2025.100771. Epub 2025 Mar 7. J Lipid Res. 2025. PMID: 40058592 Free PMC article.
References
-
- Hildebrand GD, Fielder AR. Anatomy and physiology of the Retina. In: Reynolds J, Olitsky S, editors. Pediatric retina. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 39–65.
-
- Fernandes R, Gonçalves A, Cunha-Vaz J. 6 Blood-Retinal Barrier. Ocular Drug Delivery Systems: Barriers and Application of Nanoparticulate Systems; 2012. p. 111.
Publication types
MeSH terms
Grants and funding
- GOAP/ Bayer/Global Ophthalmology Awards Program/ Bayer
- UIDB/04539/2020 and UIDP/04539/2020/Foundation for Science and Technology (FCT, Portugal)
- COMPETE-FEDER (POCI-01-0145-FEDER-007440)/Foundation for Science and Technology (FCT, Portugal)
- 2020.04811.BD/Foundation for Science and Technology (FCT, Portugal)
LinkOut - more resources
Full Text Sources
Medical

