Updated safety results from phase 3 lecanemab study in early Alzheimer's disease
- PMID: 38730496
- PMCID: PMC11084061
- DOI: 10.1186/s13195-024-01441-8
Updated safety results from phase 3 lecanemab study in early Alzheimer's disease
Erratum in
-
Correction: Updated safety results from phase 3 lecanemab study in early Alzheimer's disease.Alzheimers Res Ther. 2024 Jul 10;16(1):159. doi: 10.1186/s13195-024-01507-7. Alzheimers Res Ther. 2024. PMID: 38987826 Free PMC article. No abstract available.
Abstract
Background: Alzheimer disease (AD) is a major health problem of aging, with tremendous burden on healthcare systems, patients, and families globally. Lecanemab, an FDA-approved amyloid beta (Aβ)-directed antibody indicated for the treatment of early AD, binds with high affinity to soluble Aβ protofibrils, which have been shown to be more toxic to neurons than monomers or insoluble fibrils. Lecanemab has been shown to be well tolerated in multiple clinical trials, although risks include an increased rate of amyloid-related imaging abnormalities (ARIA) and infusion reactions relative to placebo.
Methods: Clarity AD was an 18-month treatment (Core study), multicenter, double-blind, placebo-controlled, parallel-group study with open-label extension (OLE) in participants with early AD. Eligible participants were randomized 1:1 across 2 treatment groups (placebo and lecanemab 10 mg/kg biweekly). Safety evaluations included monitoring of vital signs, physical examinations, adverse events, clinical laboratory parameters, and 12-lead electrocardiograms. ARIA occurrence was monitored throughout the study by magnetic resonance imaging, read both locally and centrally.
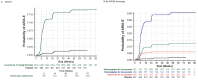
Results: Overall, 1795 participants from Core and 1612 participants with at least one dose of lecanemab (Core + OLE) were included. Lecanemab was generally well-tolerated in Clarity AD, with no deaths related to lecanemab in the Core study. There were 9 deaths during the OLE, with 4 deemed possibly related to study treatment. Of the 24 deaths in Core + OLE, 3 were due to intracerebral hemorrhage (ICH): 1 placebo in the Core due to ICH, and 2 lecanemab in OLE with concurrent ICH (1 on tissue plasminogen activator and 1 on anticoagulant therapy). In the Core + OLE, the most common adverse events in the lecanemab group (> 10%) were infusion-related reactions (24.5%), ARIA with hemosiderin deposits (ARIA-H) microhemorrhages (16.0%), COVID-19 (14.7%), ARIA with edema (ARIA-E; 13.6%), and headache (10.3%). ARIA-E and ARIA-H were largely radiographically mild-to-moderate. ARIA-E generally occurred within 3-6 months of treatment, was more common in ApoE e4 carriers (16.8%) and most common in ApoE ε4 homozygous participants (34.5%).
Conclusions: Lecanemab was generally well-tolerated, with the most common adverse events being infusion-related reactions, ARIA-H, ARIA-E. Clinicians, participants, and caregivers should understand the incidence, monitoring, and management of these events for optimal patient care.
Trial registration: ClinicalTrials.gov numbers: Clarity AD NCT03887455).
Keywords: ARIA; Alzheimer’s disease; Lecanemab; Safety.
© 2024. The Author(s).
Conflict of interest statement
LSH receives research funding from Abbvie, Acumen, Alector, Biogen, Bristol-Myers Squibb, Cognition, EIP, Eisai, Ferrer, Genentech/Roche, Janssen/J&J, Transposon, UCB, Vaccinex. LSH is a consultant for Alector, Biogen, Cortexyme, Eisai, Medscape, and Prevail/Lilly.
MNS has ownership interest (Stock or stock options): NeuroTau, uMethod Health, Versanum, Athira, TransDermix, Seq BioMarque, NeuroReserve, Cortexyme/Quince Therapeutics, Lighthouse Therapeutics. MNS is a consultant for: Alzheon, Biogen, Roche-Genentech, Eisai, KeifeRx, Lilly, Synaptogenix, NeuroTherapia, T3D, Signant Health, Novo Nordisk. MNS receives royalties from Humanix. MNS is a Board of Director for EIP Pharma.
CHvD is a consultant for Roche, Eisai, Cerevel, and Ono and receives research support from Biogen, Eisai, Roche, Genentech, Eli Lilly, Janssen, UCB, Cerevel, and Biohaven.
RAS is a consultant to: AbbVie, AC Immune, Acumen, Alector, Bristol-Myers Squibb, Genentech, Ionis, Janssen, Oligomerix, Prothena, Roche, Shionogi. RAS receives research funding from the National Institute on Aging (P01AG036694; R01AG054029; R01AG061848, U24AG057437; R01 AG03689), the Alzheimer’s Association, GHR Foundation, Eli Lilly, Eisai – Public-Private Partnership Trial Funding, and. Accelerating Medicines Partnership FNIH.
SH, AM, LG, MG, MK, DL, SD, MI, and LK are employees of Eisai.
DP is an employee of Clario.
Figures
References
-
- Alzheimer’s A. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2023;19(4). 10.1002/alz.13016. https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf Accessed November 28, 2023.
-
- Masters C, Bateman R, Blennow K, et al. Alzheimer’s disease. Nat Rev. 2015;1:15056. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous