The Causes and Consequences of DNA Damage and Chromosomal Instability Induced by Human Papillomavirus
- PMID: 38730612
- PMCID: PMC11083350
- DOI: 10.3390/cancers16091662
The Causes and Consequences of DNA Damage and Chromosomal Instability Induced by Human Papillomavirus
Abstract
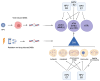
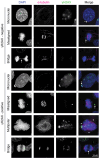
High-risk human papillomaviruses (HPVs) are the main cause of cervical, oropharyngeal, and anogenital cancers, which are all treated with definitive chemoradiation therapy when locally advanced. HPV proteins are known to exploit the host DNA damage response to enable viral replication and the epithelial differentiation protocol. This has far-reaching consequences for the host genome, as the DNA damage response is critical for the maintenance of genomic stability. HPV+ cells therefore have increased DNA damage, leading to widespread genomic instability, a hallmark of cancer, which can contribute to tumorigenesis. Following transformation, high-risk HPV oncoproteins induce chromosomal instability, or chromosome missegregation during mitosis, which is associated with a further increase in DNA damage, particularly due to micronuclei and double-strand break formation. Thus, HPV induces significant DNA damage and activation of the DNA damage response in multiple contexts, which likely affects radiation sensitivity and efficacy. Here, we review how HPV activates the DNA damage response, how it induces chromosome missegregation and micronuclei formation, and discuss how these factors may affect radiation response. Understanding how HPV affects the DNA damage response in the context of radiation therapy may help determine potential mechanisms to improve therapeutic response.
Keywords: DNA damage response; alternative end-joining; chromosomal instability (CIN); human papillomavirus (HPV); mitosis; radiation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kreisel K.M., Spicknall I.H., Gargano J.W., Lewis F.M.T., Lewis R.M., Markowitz L.E., Roberts H., Johnson A.S., Song R., St Cyr S.B., et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2018. Sex. Transm. Dis. 2021;48:208–214. doi: 10.1097/OLQ.0000000000001355. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

