Endoscopic Diagnosis of Small Bowel Tumor
- PMID: 38730658
- PMCID: PMC11083951
- DOI: 10.3390/cancers16091704
Endoscopic Diagnosis of Small Bowel Tumor
Abstract
Recent technological advances, including capsule endoscopy (CE) and balloon-assisted endoscopy (BAE), have revealed that small intestinal disease is more common than previously thought. CE has advantages, including a high diagnostic yield, discomfort-free, outpatient basis, and physiological images. BAE enabled endoscopic diagnosis and treatment in the deep small bowel. Computed tomography (CT) enterography with negative oral contrast can evaluate masses, wall thickening, and narrowing of the small intestine. In addition, enhanced CT can detect abnormalities outside the gastrointestinal tract that endoscopy cannot evaluate. Each modality has its advantages and disadvantages, and a good combination of multiple modalities leads to an accurate diagnosis. As a first-line modality, three-phase enhanced CT is preferred. If CT shows a mass, stenosis, or wall thickening, a BAE should be selected. If there are no abnormal findings on CT and no obstructive symptoms, CE should be selected. If there are significant findings in the CE, determine the indication for BAE and its insertion route based on these findings. Early diagnosis of small intestinal tumors is essential for favorable outcomes. For early diagnosis, the possibility of small bowel lesions should be considered in patients with unexplained symptoms and signs after examination of the upper and lower gastrointestinal tract.
Keywords: CT enterography; balloon-assisted enteroscopy; capsule endoscopy.
Conflict of interest statement
Hironori Yamamoto has patents for double-balloon endoscopy and a consultant relationship with Fujifilm. Tomonori Yano has received research funding and honoraria from Fujifilm.
Figures
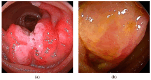
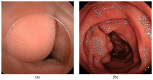
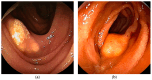
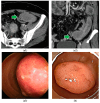
Similar articles
-
Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline.Endoscopy. 2015 Apr;47(4):352-76. doi: 10.1055/s-0034-1391855. Epub 2015 Mar 31. Endoscopy. 2015. PMID: 25826168
-
A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease.Am J Gastroenterol. 2006 May;101(5):954-64. doi: 10.1111/j.1572-0241.2006.00506.x. Am J Gastroenterol. 2006. PMID: 16696781
-
Diagnostic evaluation and management of obscure gastrointestinal bleeding: a changing paradigm.Gastroenterol Hepatol (N Y). 2009 Dec;5(12):839-50. Gastroenterol Hepatol (N Y). 2009. PMID: 20567529 Free PMC article.
-
Small bowel imaging - still a radiologic approach?Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010 Jun;154(2):123-32. doi: 10.5507/bp.2010.019. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010. PMID: 20668493 Review.
-
Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review.Endoscopy. 2018 Apr;50(4):423-446. doi: 10.1055/a-0576-0566. Epub 2018 Mar 14. Endoscopy. 2018. PMID: 29539652 Review.
Cited by
-
The Many Faces of Intestinal Tumors in Adults, Including the Primary Role of CT Imaging in Emergencies and the Important Role of Cross-Sectional Imaging: A Pictorial Review.Healthcare (Basel). 2025 May 6;13(9):1071. doi: 10.3390/healthcare13091071. Healthcare (Basel). 2025. PMID: 40361849 Free PMC article. Review.
References
-
- Yamashita K., Oka S., Yamada T., Mitsui K., Yamamoto H., Takahashi K., Shiomi A., Hotta K., Takeuchi Y., Kuwai T., et al. Clinicopathological features and prognosis of primary small bowel adenocarcinoma: A large multicenter analysis of the JSCCR database in Japan. J. Gastroenterol. 2024;59:376–388. doi: 10.1007/s00535-024-02081-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

