Adsorption of Heavy Metal Ions on Alginate-Based Magnetic Nanocomposite Adsorbent Beads
- PMID: 38730748
- PMCID: PMC11084431
- DOI: 10.3390/ma17091942
Adsorption of Heavy Metal Ions on Alginate-Based Magnetic Nanocomposite Adsorbent Beads
Abstract
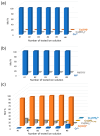
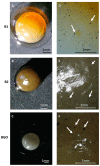
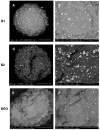
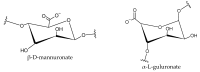
Graphene oxide and its magnetic nanoparticle-based composites are a well-known tool to remove heavy metals from wastewater. Unfortunately, one of the major issues in handling such small particles consists of their difficult removal from treated wastewater (even when their magnetic properties are exploited), due to their very small diameter. One possible way to overcome this problem is to embed them in a macroscopic biopolymer matrix, such as alginate or chitosan beads. In this way, the adsorbent becomes easier to handle and can be used to build, for example, a packed column, as in a traditional industrial adsorber. In this work, the removal performances of two different embedded magnetic nanocomposite adsorbents (MNAs) are discussed. The first type of MNA is based on ferrite magnetic nanoparticles (MNPs) generated by coprecipitation using iron(II/III) salts and ammonium hydroxide, while the second is based on a 2D material composed of MNP-decorated graphene oxide. Both MNAs were embedded in cross-linked alginate beads and used to treat artificial water contaminated with chromium(III), nickel(II), and copper(II) in different concentrations. The yield of removal and differences between MNAs and non-embedded magnetic nanomaterials are also discussed. From the results, it was found that the time to reach the adsorption equilibrium is higher when compared to that of the nanomaterials only, due to the lower surface/volume ratio of the beads, but the adsorption capacity is higher, due to the additional interaction with alginate.
Keywords: adsorption; graphene oxide; magnetic nanoparticles; nanoadsorbents; wastewater.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Wang Y., Serventi L. Sustainability of Dairy and Soy Processing: A Review on Wastewater Recycling. J. Clean. Prod. 2019;237:117821. doi: 10.1016/j.jclepro.2019.117821. - DOI
-
- Lee K.E., Morad N., Teng T.T., Poh B.T. Development, Characterization and the Application of Hybrid Materials in Coagulation/Flocculation of Wastewater: A Review. Chem. Eng. J. 2012;203:370–386. doi: 10.1016/j.cej.2012.06.109. - DOI
LinkOut - more resources
Full Text Sources

