Creation and Validation of Patient-Derived Cancer Model Using Peritoneal and Pleural Effusion in Patients with Advanced Ovarian Cancer: An Early Experience
- PMID: 38731247
- PMCID: PMC11084603
- DOI: 10.3390/jcm13092718
Creation and Validation of Patient-Derived Cancer Model Using Peritoneal and Pleural Effusion in Patients with Advanced Ovarian Cancer: An Early Experience
Abstract
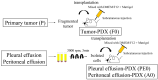
Background: The application of personalized cancer treatment based on genetic information and surgical samples has begun in the field of cancer medicine. However, a biopsy may be painful for patients with advanced diseases that do not qualify for surgical resection. Patient-derived xenografts (PDXs) are cancer models in which patient samples are transplanted into immunodeficient mice. PDXs are expected to be useful for personalized medicine. The aim of this study was to establish a PDX from body fluid (PDX-BF), such as peritoneal and pleural effusion samples, to provide personalized medicine without surgery. Methods: PDXs-BF were created from patients with ovarian cancer who had positive cytology findings based on peritoneal and pleural effusion samples. PDXs were also prepared from each primary tumor. The pathological findings based on immunohistochemistry were compared between the primary tumor, PDX, and PDX-BF. Further, genomic profiles and gene expression were evaluated using DNA and RNA sequencing to compare primary tumors, PDXs, and PDX-BF. Results: Among the 15 patients, PDX-BF was established for 8 patients (5 high-grade serous carcinoma, 1 carcinosarcoma, 1 low-grade serous carcinoma, and 1 clear cell carcinoma); the success rate was 53%. Histologically, PDXs-BF have features similar to those of primary tumors and PDXs. In particular, PDXs-BF had similar gene mutations and expression patterns to primary tumors and PDXs. Conclusions: PDX-BF reproduced primary tumors in terms of pathological features and genomic profiles, including gene mutation and expression. Thus, PDX-BF may be a potential alternative to surgical resection for patients with advanced disease.
Keywords: ascites; ovarian cancer; patient-derived xenograft; pleural effusion; sequence analysis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Nunes M., Ricardo S. Chemoresistance in Ovarian Cancer: The Role of Malignant Ascites. In: Lele S., editor. Ovarian Cancer. Exon Publications; Brisbane, AU, Australia: 2022. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

