The Benzoylpiperidine Fragment as a Privileged Structure in Medicinal Chemistry: A Comprehensive Review
- PMID: 38731421
- PMCID: PMC11085656
- DOI: 10.3390/molecules29091930
The Benzoylpiperidine Fragment as a Privileged Structure in Medicinal Chemistry: A Comprehensive Review
Abstract
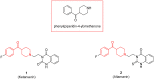
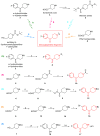
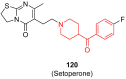
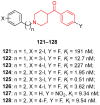
The phenyl(piperidin-4-yl)methanone fragment (here referred to as the benzoylpiperidine fragment) is a privileged structure in the development of new drugs considering its presence in many bioactive small molecules with both therapeutic (such as anti-cancer, anti-psychotic, anti-thrombotic, anti-arrhythmic, anti-tubercular, anti-parasitic, anti-diabetic, and neuroprotective agents) and diagnostic properties. The benzoylpiperidine fragment is metabolically stable, and it is also considered a potential bioisostere of the piperazine ring, thus making it a feasible and reliable chemical frame to be exploited in drug design. Herein, we discuss the main therapeutic and diagnostic agents presenting the benzoylpiperidine motif in their structure, covering articles reported in the literature since 2000. A specific section is focused on the synthetic strategies adopted to obtain this versatile chemical portion.
Keywords: benzoylpiperidine; benzoylpiperidine-based small molecules; bioisostere; diagnostic agents; drug design; medicinal chemistry; phenyl(piperidin-4-yl)methanone; privileged structure; therapeutic agents.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

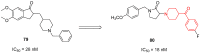
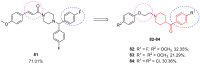
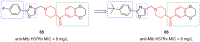
Similar articles
-
Synthesis of Novel Aryloxyethylamine Derivatives and Evaluation of Their in Vitro and in Vivo Neuroprotective Activities.Chem Biodivers. 2020 Sep;17(9):e2000431. doi: 10.1002/cbdv.202000431. Epub 2020 Sep 13. Chem Biodivers. 2020. PMID: 32583520
-
Current Fragment-to-lead Approaches Starting from the 7-azaindole: The Pharmacological Versatility of a Privileged Molecular Fragment.Curr Top Med Chem. 2023;23(22):2116-2130. doi: 10.2174/1568026623666230718100541. Curr Top Med Chem. 2023. PMID: 37461366 Review.
-
p-Benzoquinone as a Privileged Scaffold of Pharmacological Significance: A Review.Mini Rev Med Chem. 2020;20(16):1586-1609. doi: 10.2174/1389557520666200429101451. Mini Rev Med Chem. 2020. PMID: 32348217 Review.
-
The Bioactivity of Thiazolidin-4-Ones: A Short Review of the Most Recent Studies.Int J Mol Sci. 2021 Oct 26;22(21):11533. doi: 10.3390/ijms222111533. Int J Mol Sci. 2021. PMID: 34768964 Free PMC article. Review.
-
Ligustrazine as a multitarget scaffold in drug design and discovery.Bioorg Med Chem. 2025 Apr 15;121:118110. doi: 10.1016/j.bmc.2025.118110. Epub 2025 Feb 11. Bioorg Med Chem. 2025. PMID: 39955802 Review.
References
-
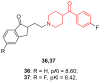
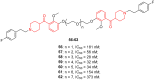
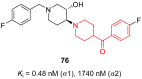
- Liu Y., Guo L., Duan H., Zhang L., Jiang N., Zhen X., Shen J. Discovery of 4-Benzoylpiperidine and 3-(Piperidin-4-Yl)Benzo[d]Isoxazole Derivatives as Potential and Selective GlyT1 Inhibitors. RSC Adv. 2015;5:40964–40977. doi: 10.1039/C5RA04714E. - DOI
-
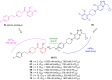
- Yadav V.D., Boshoff H.I., Trifonov L., Roma J.S.O., Ioerger T.R., Barry C.E., Oh S. Synthesis and Structure–Activity Relationships of a New Class of Oxadiazoles Targeting DprE1 as Antitubercular Agents. ACS Med. Chem. Lett. 2023;14:1275–1283. doi: 10.1021/acsmedchemlett.3c00295. - DOI - PMC - PubMed
-
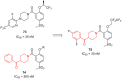
- Karmacharya U., Chaudhary P., Lim D., Dahal S., Awasthi B.P., Park H.D., Kim J.-A., Jeong B.-S. Synthesis and Anticancer Evaluation of 6-Azacyclonol-2,4,6-Trimethylpyridin-3-Ol Derivatives: M3 Muscarinic Acetylcholine Receptor-Mediated Anticancer Activity of a Cyclohexyl Derivative in Androgen-Refractory Prostate Cancer. Bioorg. Chem. 2021;110:104805. doi: 10.1016/j.bioorg.2021.104805. - DOI - PubMed
-
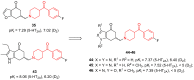
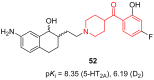
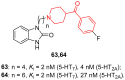
- Jin J., Zhang K., Dou F., Hao C., Zhang Y., Cao X., Gao L., Xiong J., Liu X., Liu B.-F., et al. Isoquinolinone Derivatives as Potent CNS Multi-Receptor D2/5-HT1A/5-HT2A/5-HT6/5-HT7 Agents: Synthesis and Pharmacological Evaluation. Eur. J. Med. Chem. 2020;207:112709. doi: 10.1016/j.ejmech.2020.112709. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

