Two Fluorescent Probes for Recognition of Acetylcholinesterase: Design, Synthesis, and Comparative Evaluation
- PMID: 38731452
- PMCID: PMC11085145
- DOI: 10.3390/molecules29091961
Two Fluorescent Probes for Recognition of Acetylcholinesterase: Design, Synthesis, and Comparative Evaluation
Abstract
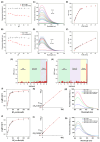
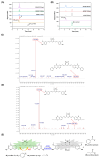
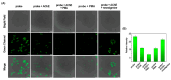
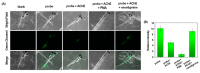
In this study, two "on-off" probes (BF2-cur-Ben and BF2-cur-But) recognizing acetylcholinesterase (AChE) were designed and synthesized. The obtained probes can achieve recognition of AChE with good selectivity and pH-independence with a linear range of 0.5~7 U/mL and 0.5~25 U/mL respectively. BF2-cur-Ben has a lower limit of detection (LOD) (0.031 U/mL), higher enzyme affinity (Km = 16 ± 1.6 μM), and higher inhibitor sensitivity. A responsive mechanism of the probes for AChE was proposed based on HPLC and mass spectra (MS) experiments, as well as calculations. In molecular simulation, BF2-cur-Ben forms more hydrogen bonds (seven, while BF2-cur-But has only four) and thus has a more stable enzyme affinity, which is mirrored by the results of the comparison of Km values. These two probes could enable recognition of intracellular AChE and probe BF2-cur-Ben has superior cell membrane penetration due to its higher log p value. These probes can monitor the overexpression of AChE during apoptosis of lung cancer cells. The ability of BF2-cur-Ben to monitor AChE in vivo was confirmed by a zebrafish experiment.
Keywords: acetylcholinesterase; apoptosis; comparative evaluation; lung carcinoma cell; probe; visualized monitoring.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Sola I., Aso E., Frattini D., López-González I., Espargaró A., Sabaté R., Di Pietro O., Luque F.J., Clos M.V., Ferrer I., et al. Novel Levetiracetam Derivatives That Are Effective against the Alzheimer-like Phenotype in Mice: Synthesis, in Vitro, ex Vivo, and in Vivo Efficacy Studies. J. Med. Chem. 2015;58:6018–6032. doi: 10.1021/acs.jmedchem.5b00624. - DOI - PubMed
-
- Oukoloff K., Chao S., CieslikiewiczBouet M., Mougeot R., Jean L., Renard P.Y. Improved Access to Huprine Derivatives Functionalized at Position 9. Eur. J. Org. Chem. 2016;2016:1337–1343. doi: 10.1002/ejoc.201501433. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 2022GXNSFAA035453/Guangxi Natural Science Foundation
- 22267002/National Natural Science Foundation of China
- GYJK (2023)003/Drug safety research project of Guangxi Zhuang Autonomous Region Drug Administration
- Z-A20221011/Self-financed scientific research of Guangxi Zhuang Autonomous Region Health Commission in 2022
- 2022KY1387/2022 Guangxi Higher Education Institutions Young and Middle-aged Teachers Scientific Research Basic Ability Enhancement Project
LinkOut - more resources
Full Text Sources
Miscellaneous

