Verification of In Vitro Anticancer Activity and Bioactive Compounds in Cordyceps Militaris-Infused Sweet Potato Shochu Spirits
- PMID: 38731610
- PMCID: PMC11085083
- DOI: 10.3390/molecules29092119
Verification of In Vitro Anticancer Activity and Bioactive Compounds in Cordyceps Militaris-Infused Sweet Potato Shochu Spirits
Abstract
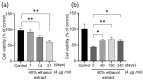
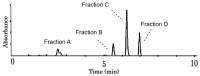
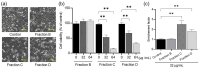
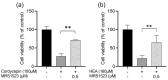
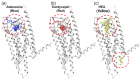
Many liqueurs, including spirits infused with botanicals, are crafted not only for their taste and flavor but also for potential medicinal benefits. However, the scientific evidence supporting their medicinal effects remains limited. This study aims to verify in vitro anticancer activity and bioactive compounds in shochu spirits infused with Cordyceps militaris, a Chinese medicine. The results revealed that a bioactive fraction was eluted from the spirit extract with 40% ethanol. The infusion time impacted the inhibitory effect of the spirit extract on the proliferation of colon cancer-derived cell line HCT-116 cells, and a 21-day infusion showed the strongest inhibitory effect. Furthermore, the spirit extract was separated into four fractions, A-D, by high-performance liquid chromatography (HPLC), and Fractions B, C, and D, but not A, exerted the effects of proliferation inhibition and apoptotic induction of HCT-116 cells and HL-60 cells. Furthermore, Fractions B, C, and D were, respectively, identified as adenosine, cordycepin, and N6-(2-hydroxyethyl)-adenosine (HEA) by comprehensive chemical analyses, including proton nuclear magnetic resonance (1H-NMR), Fourier transform infrared spectroscopy (FT-IR), and electrospray ionization mass spectrometry (ESI-MS). To better understand the bioactivity mechanisms of cordycepin and HEA, the agonist and antagonist tests of the A3 adenosine receptor (A3AR) were performed. Cell viability was suppressed by cordycepin, and HEA was restored by the A3AR antagonist MR1523, suggesting that cordycepin and HEA possibly acted as agonists to activate A3ARs to inhibit cell proliferation. Molecular docking simulations revealed that both adenosine and cordycepin bound to the same pocket site of A3ARs, while HEA exhibited a different binding pattern, supporting a possible explanation for the difference in their bioactivity. Taken together, the present study demonstrated that cordycepin and HEA were major bioactive ingredients in Cordyceps militaries-infused sweet potato shochu spirits, which contributed to the in vitro anticancer activity.
Keywords: A3 adenosine receptor; N6-(2-hydroxyethyl)-adenosine; Shochu spirits; adenosine derivatives; anticancer activity; cordycepin; cordyceps militaris.
Conflict of interest statement
Cho Sho is employed by the company Kirishima Shuzo Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



Similar articles
-
Preparative isolation of cordycepin, N(6)-(2-hydroxyethyl)-adenosine and adenosine from Cordyceps militaris by macroporous resin and purification by recycling high-speed counter-current chromatography.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Oct 15;1033-1034:218-225. doi: 10.1016/j.jchromb.2016.08.025. Epub 2016 Aug 16. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 27567378
-
Integrating Network Pharmacology and Optimization of Preparation Methods to Enhance the Anticancer Effect of Cordyceps militaris on Lung Cancer.Anticancer Res. 2025 Jul;45(7):2963-2984. doi: 10.21873/anticanres.17663. Anticancer Res. 2025. PMID: 40578945
-
Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors.Tumour Biol. 2017 Jul;39(7):1010428317706915. doi: 10.1177/1010428317706915. Tumour Biol. 2017. PMID: 28714368
-
Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis.J Pharmacol Sci. 2015 Jan;127(1):53-6. doi: 10.1016/j.jphs.2014.09.001. Epub 2014 Oct 2. J Pharmacol Sci. 2015. PMID: 25704018 Review.
-
The Anticancer Properties of Cordycepin and Their Underlying Mechanisms.Int J Mol Sci. 2018 Oct 4;19(10):3027. doi: 10.3390/ijms19103027. Int J Mol Sci. 2018. PMID: 30287757 Free PMC article. Review.
Cited by
-
Entomopathogenic fungi: insights into recent understanding.World J Microbiol Biotechnol. 2025 May 26;41(6):179. doi: 10.1007/s11274-025-04377-9. World J Microbiol Biotechnol. 2025. PMID: 40415063 Review.
References
-
- Rodríguez-Solana R., Vázquez-Araújo L., Salgado J.M., Domínguez J.M., Cortés-Diéguez S. Optimization of the process of aromatic and medicinal plant maceration in grape marc distillates to obtain herbal liqueurs and spirits. J. Sci. Food Agric. 2016;96:4760–4771. doi: 10.1002/jsfa.7822. - DOI - PubMed
-
- Yahagi N. Illustrated catalogue of Japanese Cordyceps (entomogenous fungi): The Yahagi collection of Japanese Cordyceps stored in the Tohoku University Museum. Bull. Tohoku Univ. Mus. 2008;8:29–89.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

