Coordination Polymer Based on a Triangular Carboxylate Core {Fe(μ3-O)(μ-O2CR)6} and an Aliphatic Diamine
- PMID: 38731615
- PMCID: PMC11085704
- DOI: 10.3390/molecules29092125
Coordination Polymer Based on a Triangular Carboxylate Core {Fe(μ3-O)(μ-O2CR)6} and an Aliphatic Diamine
Abstract
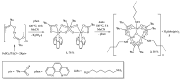
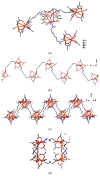
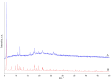
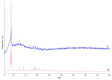
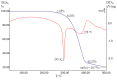
Interaction of the pre-organized complex of iron(II) trimethylacetate and 1,10-phenanthroline (phen) [Fe2(piv)4(phen)2] (1) (piv = (Me)3CCO2-)) with 1,6-diaminohexane (dahx) in anhydrous acetonitrile yielded a 1D coordination polymer [Fe3O(piv)6(dahx)1.5]n (2) and an organic salt of pivalic acid (H2dahx)(piv)2 (3). The structure of the obtained compounds was determined by single-crystal X-ray diffraction analysis. The phase purity of the complexes was determined by powder X-ray diffraction analysis. According to the single-crystal X-ray analysis, coordination polymer 2 is formed due to the binding of a triangular carboxylate core {Fe3(μ3-O)(μ-piv)6} with an aliphatic diamine ligand. Thermal behavior was investigated for compounds 1 and 2 in an argon atmosphere.
Keywords: aliphatic diamines; carboxylate complexes; coordination polymers; iron complexes; molecular structure.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Agafonov M.A., Alexandrov E.V., Artyukhova N.A., Bekmukhamedov G.E., Blatov V.A., Butova V.V., Gayfulin Y.M., Garibyan A.A., Gafurov Z.N., Gorbunova Y.G., et al. Metal-Organic Frameworks in Russia: From the Synthesis and Structure to Functional Properties and Materials. J. Struct. Chem. 2022;63:671–843. doi: 10.1134/S0022476622050018. - DOI
-
- Lytvynenko A.S., Kolotilov S.V., Kiskin M.A., Cador O., Golhen S., Aleksandrov G.G., Mishura A.M., Titov V.E., Ouahab L., Eremenko I.L., et al. Redox-Active Porous Coordination Polymers Prepared by Trinuclear Heterometallic Pivalate Linking with the Redox-Active Nickel(II) Complex: Synthesis, Structure, Magnetic and Redox Properties, and Electrocatalytic Activity in Organic Compound Dehalogenatio. Inorg. Chem. 2014;53:4970–4979. doi: 10.1021/ic403167m. - DOI - PubMed
-
- Polunin R.A., Kolotilov S.V., Kiskin M.A., Cador O., Golhen S., Shvets O.V., Ouahab L., Dobrokhotova Z.V., Ovcharenko V.I., Eremenko I.L., et al. V Structural Flexibility and Sorption Properties of 2D Porous Coordination Polymers Constructed from Trinuclear Heterometallic Pivalates and 4,4′-Bipyridine. Eur. J. Inorg. Chem. 2011;2011:4985–4992. doi: 10.1002/ejic.201100791. - DOI
-
- Petrov P.A., Nikolaevskii S.A., Yambulatov D.S., Starikova A.A., Sukhikh T.S., Kiskin M.A., Sokolov M.N., Eremenko I.L. Heteroleptic Anionic Cobalt(II) Pivalate Complex with a Bridging Trimethylsiloxy Ligand: Synthesis, Structure, and Formation Mechanism. Russ. J. Inorg. Chem. 2023;68:1255–1264. doi: 10.1134/S0036023623601460. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

