Indole-Based Compounds in the Development of Anti-Neurodegenerative Agents
- PMID: 38731618
- PMCID: PMC11085553
- DOI: 10.3390/molecules29092127
Indole-Based Compounds in the Development of Anti-Neurodegenerative Agents
Abstract
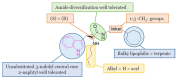
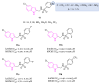
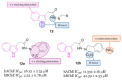
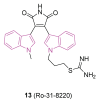
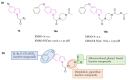
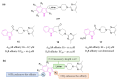
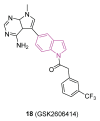
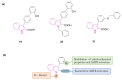
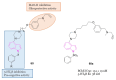
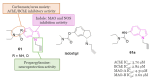
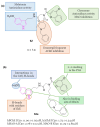
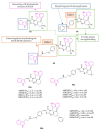
Neurodegeneration is a gradual decay process leading to the depletion of neurons in both the central and peripheral nervous systems, ultimately resulting in cognitive dysfunctions and the deterioration of brain functions, alongside a decline in motor skills and behavioral capabilities. Neurodegenerative disorders (NDs) impose a substantial socio-economic strain on society, aggravated by the advancing age of the world population and the absence of effective remedies, predicting a negative future. In this context, the urgency of discovering viable therapies is critical and, despite significant efforts by medicinal chemists in developing potential drug candidates and exploring various small molecules as therapeutics, regrettably, a truly effective treatment is yet to be found. Nitrogen heterocyclic compounds, and particularly those containing the indole nucleus, which has emerged as privileged scaffold, have attracted particular attention for a variety of pharmacological applications. This review analyzes the rational design strategy adopted by different research groups for the development of anti-neurodegenerative indole-based compounds which have the potential to modulate various molecular targets involved in NDs, with reference to the most recent advances between 2018 and 2023.
Keywords: indole nucleus; multifunctional compounds; neurodegeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
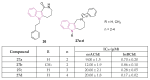
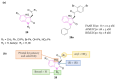
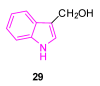
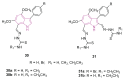

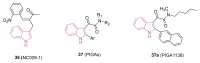
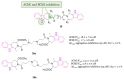
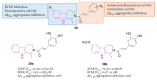
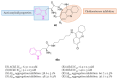
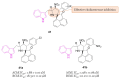
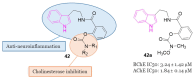
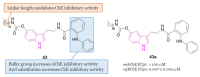
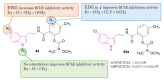
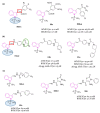
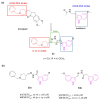
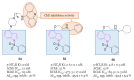
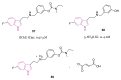
References
-
- Duarte Y., Fonseca A., Gutiérrez M., Adasme-Carreño F., Muñoz-Gutierrez C., Alzate-Morales J., Santana L., Uriarte E., Álvarez R., Matos M.J. Novel Coumarin-Quinoline Hybrids: Design of Multitarget Compounds for Alzheimer’s Disease. ChemistrySelect. 2019;4:551–558. doi: 10.1002/slct.201803222. - DOI
-
- Chauhan M.S.S., Umar T., Aulakh M.K. Quinolines: Privileged Scaffolds for Developing New Anti-Neurodegenerative Agents. ChemistrySelect. 2023;8:e202204960. doi: 10.1002/slct.202204960. - DOI
-
- Savelieff M.G., Nam G., Kang J., Lee H.J., Lee M., Lim M.H. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019;119:1221–1322. doi: 10.1021/acs.chemrev.8b00138. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

