Maternal Diet High in Linoleic Acid Alters Renal Branching Morphogenesis and mTOR/AKT Signalling Genes in Rat Fetal Kidneys
- PMID: 38731907
- PMCID: PMC11083378
- DOI: 10.3390/ijms25094688
Maternal Diet High in Linoleic Acid Alters Renal Branching Morphogenesis and mTOR/AKT Signalling Genes in Rat Fetal Kidneys
Abstract
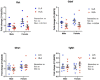
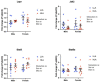
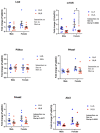
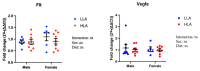
Linoleic acid (LA), an n-6 polyunsaturated fatty acid (PUFA), is obtained from the maternal diet during pregnancy, and is essential for normal fetal growth and development. A maternal high-LA (HLA) diet alters maternal and offspring fatty acids, maternal leptin and male/female ratio at embryonic (E) day 20 (E20). We investigated the effects of an HLA diet on embryonic offspring renal branching morphogenesis, leptin signalling, megalin signalling and angiogenesis gene expression. Female Wistar Kyoto rats were fed low-LA (LLA; 1.44% energy from LA) or high-LA (HLA; 6.21% energy from LA) diets during pregnancy and gestation/lactation. Offspring were sacrificed and mRNA from kidneys was analysed by real-time PCR. Maternal HLA decreased the targets involved in branching morphogenesis Ret and Gdnf in offspring, independent of sex. Furthermore, downstream targets of megalin, namely mTOR, Akt3 and Prkab2, were reduced in offspring from mothers consuming an HLA diet, independent of sex. There was a trend of an increase in the branching morphogenesis target Gfra1 in females (p = 0.0517). These findings suggest that an HLA diet during pregnancy may lead to altered renal function in offspring. Future research should investigate the effects an HLA diet has on offspring kidney function in adolescence and adulthood.
Keywords: kidney; linoleic acid; maternal; offspring; sex specific.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ramsden C.E., Ringel A., Feldstein A.E., Taha A.Y., MacIntosh B.A., Hibbeln J.R., Majchrzak-Hong S.F., Faurot K.R., Rapoport S.I., Cheon Y., et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot. Essent. Fat. Acids. 2012;87:135–141. doi: 10.1016/j.plefa.2012.08.004. - DOI - PMC - PubMed
-
- Ailhaud G., Massiera F., Weill P., Legrand P., Alessandri J.M., Guesnet P. Temporal changes in dietary fats: Role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 2006;45:203–236. doi: 10.1016/j.plipres.2006.01.003. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

