Functional and Genetic Analyses Unveil the Implication of CDC27 in Hemifacial Microsomia
- PMID: 38731925
- PMCID: PMC11083823
- DOI: 10.3390/ijms25094707
Functional and Genetic Analyses Unveil the Implication of CDC27 in Hemifacial Microsomia
Abstract
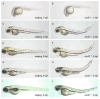
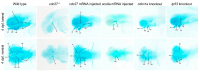
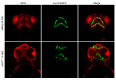
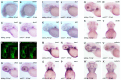
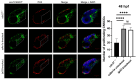
Hemifacial microsomia (HFM) is a rare congenital genetic syndrome primarily affecting the first and second pharyngeal arches, leading to defects in the mandible, external ear, and middle ear. The pathogenic genes remain largely unidentified. Whole-exome sequencing (WES) was conducted on 12 HFM probands and their unaffected biological parents. Predictive structural analysis of the target gene was conducted using PSIPRED (v3.3) and SWISS-MODEL, while STRING facilitated protein-to-protein interaction predictions. CRISPR/Cas9 was applied for gene knockout in zebrafish. In situ hybridization (ISH) was employed to examine the spatiotemporal expression of the target gene and neural crest cell (NCC) markers. Immunofluorescence with PH3 and TUNEL assays were used to assess cell proliferation and apoptosis. RNA sequencing was performed on mutant and control embryos, with rescue experiments involving target mRNA injections and specific gene knockouts. CDC27 was identified as a novel candidate gene for HFM, with four nonsynonymous de novo variants detected in three unrelated probands. Structural predictions indicated significant alterations in the secondary and tertiary structures of CDC27. cdc27 knockout in zebrafish resulted in craniofacial malformation, spine deformity, and cardiac edema, mirroring typical HFM phenotypes. Abnormalities in somatic cell apoptosis, reduced NCC proliferation in pharyngeal arches, and chondrocyte differentiation issues were observed in cdc27-/- mutants. cdc27 mRNA injections and cdkn1a or tp53 knockout significantly rescued pharyngeal arch cartilage dysplasia, while sox9a mRNA administration partially restored the defective phenotypes. Our findings suggest a functional link between CDC27 and HFM, primarily through the inhibition of CNCC proliferation and disruption of pharyngeal chondrocyte differentiation.
Keywords: CDC27; CRISPR/Cas9; hemifacial microsomia; neural crest cell; rescue experiments; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
ctdsp2 Knockout Induces Zebrafish Craniofacial Dysplasia via p53 Signaling Activation.Int J Mol Sci. 2025 Feb 3;26(3):1297. doi: 10.3390/ijms26031297. Int J Mol Sci. 2025. PMID: 39941065 Free PMC article.
-
FBLN2 is associated with Goldenhar syndrome and is essential for cranial neural crest cell development.Ann N Y Acad Sci. 2024 Jul;1537(1):113-128. doi: 10.1111/nyas.15183. Epub 2024 Jul 6. Ann N Y Acad Sci. 2024. PMID: 38970771
-
A Mutation in VWA1, Encoding von Willebrand Factor A Domain-Containing Protein 1, Is Associated With Hemifacial Microsomia.Front Cell Dev Biol. 2020 Sep 9;8:571004. doi: 10.3389/fcell.2020.571004. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015062 Free PMC article.
-
Etiology and Pathogenesis of Hemifacial Microsomia.J Dent Res. 2018 Nov;97(12):1297-1305. doi: 10.1177/0022034518795609. Epub 2018 Sep 11. J Dent Res. 2018. PMID: 30205013 Review.
-
The etiology, clinical features, and treatment options of hemifacial microsomia.Oral Dis. 2023 Sep;29(6):2449-2462. doi: 10.1111/odi.14508. Epub 2023 Mar 13. Oral Dis. 2023. PMID: 36648381 Review.
Cited by
-
ctdsp2 Knockout Induces Zebrafish Craniofacial Dysplasia via p53 Signaling Activation.Int J Mol Sci. 2025 Feb 3;26(3):1297. doi: 10.3390/ijms26031297. Int J Mol Sci. 2025. PMID: 39941065 Free PMC article.
References
-
- Tasse C., Böhringer S., Fischer S., Lüdecke H.J., Albrecht B., Horn D., Janecke A., Kling R., König R., Lorenz B., et al. Oculo-auriculo-vertebral spectrum (OAVS): Clinical evaluation and severity scoring of 53 patients and proposal for a new classification. Eur. J. Med. Genet. 2005;48:397–411. doi: 10.1016/j.ejmg.2005.04.015. - DOI - PubMed
-
- Wang Y., Ping L., Luan X., Chen Y., Fan X., Li L., Liu Y., Wang P., Zhang S., Zhang B., et al. A Mutation in VWA1, Encoding von Willebrand Factor A Domain-Containing Protein 1, Is Associated with Hemifacial Microsomia. Front. Cell Dev. Biol. 2020;8:571004. doi: 10.3389/fcell.2020.571004. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

