Novel Scorpion Toxin ω-Buthitoxin-Hf1a Selectively Inhibits Calcium Influx via CaV3.3 and CaV3.2 and Alleviates Allodynia in a Mouse Model of Acute Postsurgical Pain
- PMID: 38731963
- PMCID: PMC11084959
- DOI: 10.3390/ijms25094745
Novel Scorpion Toxin ω-Buthitoxin-Hf1a Selectively Inhibits Calcium Influx via CaV3.3 and CaV3.2 and Alleviates Allodynia in a Mouse Model of Acute Postsurgical Pain
Abstract
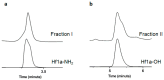
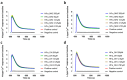
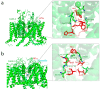
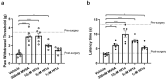
Venom peptides have evolved to target a wide range of membrane proteins through diverse mechanisms of action and structures, providing promising therapeutic leads for diseases, including pain, epilepsy, and cancer, as well as unique probes of ion channel structure-function. In this work, a high-throughput FLIPR window current screening assay on T-type CaV3.2 guided the isolation of a novel peptide named ω-Buthitoxin-Hf1a from scorpion Hottentotta franzwerneri crude venom. At only 10 amino acid residues with one disulfide bond, it is not only the smallest venom peptide known to target T-type CaVs but also the smallest structured scorpion venom peptide yet discovered. Synthetic Hf1a peptides were prepared with C-terminal amidation (Hf1a-NH2) or a free C-terminus (Hf1a-OH). Electrophysiological characterization revealed Hf1a-NH2 to be a concentration-dependent partial inhibitor of CaV3.2 (IC50 = 1.18 μM) and CaV3.3 (IC50 = 0.49 μM) depolarized currents but was ineffective at CaV3.1. Hf1a-OH did not show activity against any of the three T-type subtypes. Additionally, neither form showed activity against N-type CaV2.2 or L-type calcium channels. The three-dimensional structure of Hf1a-NH2 was determined using NMR spectroscopy and used in docking studies to predict its binding site at CaV3.2 and CaV3.3. As both CaV3.2 and CaV3.3 have been implicated in peripheral pain signaling, the analgesic potential of Hf1a-NH2 was explored in vivo in a mouse model of incision-induced acute post-surgical pain. Consistent with this role, Hf1a-NH2 produced antiallodynia in both mechanical and thermal pain.
Keywords: T-type calcium channels; acute post-surgical pain; peripheral pain; venom peptides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Blockade of T-type calcium channels by 6-prenylnaringenin, a hop component, alleviates neuropathic and visceral pain in mice.Neuropharmacology. 2018 Aug;138:232-244. doi: 10.1016/j.neuropharm.2018.06.020. Epub 2018 Jun 18. Neuropharmacology. 2018. PMID: 29913186
-
Therapeutic potential of RQ-00311651, a novel T-type Ca2+ channel blocker, in distinct rodent models for neuropathic and visceral pain.Pain. 2016 Aug;157(8):1655-1665. doi: 10.1097/j.pain.0000000000000565. Pain. 2016. PMID: 27023424
-
T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids.J Neurosci. 2009 Oct 21;29(42):13106-14. doi: 10.1523/JNEUROSCI.2919-09.2009. J Neurosci. 2009. PMID: 19846698 Free PMC article.
-
Characterization of the gating brake in the I-II loop of CaV3 T-type calcium channels.Channels (Austin). 2010 Nov-Dec;4(6):453-8. doi: 10.4161/chan.4.6.12889. Epub 2010 Nov 1. Channels (Austin). 2010. PMID: 21099341 Free PMC article. Review.
-
Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: news from the world of knockout mice.Am J Physiol Regul Integr Comp Physiol. 2015 Feb 15;308(4):R227-37. doi: 10.1152/ajpregu.00276.2014. Epub 2014 Dec 17. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25519728 Review.
Cited by
-
Deciphering Scorpion Toxin-Induced Pain: Molecular Mechanisms and Ion Channel Dynamics.Int J Biol Sci. 2025 Apr 21;21(7):2921-2934. doi: 10.7150/ijbs.109713. eCollection 2025. Int J Biol Sci. 2025. PMID: 40384871 Free PMC article. Review.
-
Proteomic characterization and lethality of the venom of the Black Judean scorpion, Hottentotta judaicus (Buthidae): expanded toxin diversity and revisited toxicological significance.Arch Toxicol. 2025 Sep 10. doi: 10.1007/s00204-025-04186-x. Online ahead of print. Arch Toxicol. 2025. PMID: 40928535
References
-
- Rein J.O. The Scorpion Files. Norwegian University of Science and Technology. 2009. [(accessed on 1 December 2023)]. Available online: https://www.ntnu.no/ub/scorpion-files/
-
- Tytgat J., Chandy K.G., Garcia M.L., Gutman G.A., Martin-Eauclaire M.-F., van der Walt J.J., Possani L.D. A unified nomenclature for short-chain peptides isolated from scorpion venoms: α-KTx molecular subfamilies. Trends Pharmacol. Sci. 1999;20:444–447. doi: 10.1016/S0165-6147(99)01398-X. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 21KJB350023/The Natural Science Foundation of the Jiangsu Higher Education Institutions of China
- 20JDG075/Senior Talent Foundation of Jiangsu University
- JSSCBS20210960/Innovative and Entrepreneurial Program of Jiangsu Province
- APP1072113/National Health and Medical Research Council
- FT190100482/Australian Research Council Future Fellowship
LinkOut - more resources
Full Text Sources
Research Materials

