A Structural In Silico Analysis of the Immunogenicity of L-Asparaginase from Penicillium cerradense
- PMID: 38732010
- PMCID: PMC11084778
- DOI: 10.3390/ijms25094788
A Structural In Silico Analysis of the Immunogenicity of L-Asparaginase from Penicillium cerradense
Abstract
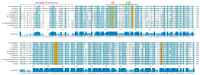
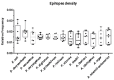
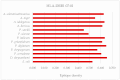
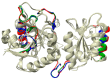
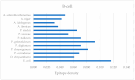
L-asparaginase is an essential drug used to treat acute lymphoid leukemia (ALL), a cancer of high prevalence in children. Several adverse reactions associated with L-asparaginase have been observed, mainly caused by immunogenicity and allergenicity. Some strategies have been adopted, such as searching for new microorganisms that produce the enzyme and applying protein engineering. Therefore, this work aimed to elucidate the molecular structure and predict the immunogenic profile of L-asparaginase from Penicillium cerradense, recently revealed as a new fungus of the genus Penicillium and producer of the enzyme, as a motivation to search for alternatives to bacterial L-asparaginase. In the evolutionary relationship, L-asparaginase from P. cerradense closely matches Aspergillus species. Using in silico tools, we characterized the enzyme as a protein fragment of 378 amino acids (39 kDa), including a signal peptide containing 17 amino acids, and the isoelectric point at 5.13. The oligomeric state was predicted to be a homotetramer. Also, this L-asparaginase presented a similar immunogenicity response (T- and B-cell epitopes) compared to Escherichia coli and Dickeya chrysanthemi enzymes. These results suggest a potentially useful L-asparaginase, with insights that can drive strategies to improve enzyme production.
Keywords: ALL; L-asparaginase; Penicillium cerradense; immunogenicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

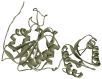
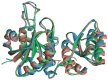



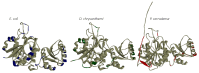
Similar articles
-
Decreasing the immunogenicity of Erwinia chrysanthemi asparaginase via protein engineering: computational approach.Mol Biol Rep. 2019 Oct;46(5):4751-4761. doi: 10.1007/s11033-019-04921-5. Epub 2019 Jul 9. Mol Biol Rep. 2019. PMID: 31290058
-
In silico identification and characterization of antineoplastic asparaginase enzyme from endophytic bacteria.IUBMB Life. 2020 May;72(5):991-1000. doi: 10.1002/iub.2237. Epub 2020 Jan 25. IUBMB Life. 2020. PMID: 31981306
-
Rhizobium etli asparaginase II: an alternative for acute lymphoblastic leukemia (ALL) treatment.Bioengineered. 2013 Jan-Feb;4(1):30-6. doi: 10.4161/bioe.21710. Epub 2012 Aug 16. Bioengineered. 2013. PMID: 22895060 Free PMC article.
-
A comprehensive review on microbial l-asparaginase: Bioprocessing, characterization, and industrial applications.Biotechnol Appl Biochem. 2020 Jul;67(4):619-647. doi: 10.1002/bab.1888. Epub 2020 Feb 18. Biotechnol Appl Biochem. 2020. PMID: 31954377 Review.
-
Can recombinant technology address asparaginase Erwinia chrysanthemi shortages?Pediatr Blood Cancer. 2021 Oct;68(10):e29169. doi: 10.1002/pbc.29169. Epub 2021 Jun 8. Pediatr Blood Cancer. 2021. PMID: 34105243 Review.
Cited by
-
Enhancing the Stability and Anticancer Activity of Escherichia coli Asparaginase Through Nanoparticle Immobilization: A Biotechnological Perspective on Nano Chitosan.Polymers (Basel). 2024 Nov 23;16(23):3260. doi: 10.3390/polym16233260. Polymers (Basel). 2024. PMID: 39684005 Free PMC article.
-
Nanomedicines Targeting Metabolic Pathways in the Tumor Microenvironment: Future Perspectives and the Role of AI.Metabolites. 2025 Mar 13;15(3):201. doi: 10.3390/metabo15030201. Metabolites. 2025. PMID: 40137165 Free PMC article. Review.
References
-
- Bon E., Corvo L., Vermelho A., Paiva C.L.A., Ferrara M., Coelho R., Alencastro R. Enzimas em Biotecnologia: Produção, Aplicações e Mercado. Interciência; Rio de Janeiro, Brazil: 2008.
-
- Van Trimpont M., Peeters E., De Visser Y., Schalk A.M., Mondelaers V., De Moerloose B., Lavie A., Lammens T., Goossens S., Van Vlierberghe P. Novel Insights on the Use of L-Asparaginase as an Efficient and Safe Anti-Cancer Therapy. Cancers. 2022;14:902. doi: 10.3390/cancers14040902. - DOI - PMC - PubMed
-
- INCA Leucemia. [(accessed on 25 March 2023)];2022 Available online: https://www.inca.gov.br/tipos-de-cancer/leucemia.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

