Gal f-Specific Neolectins: Towards Promising Diagnostic Tools
- PMID: 38732045
- PMCID: PMC11084152
- DOI: 10.3390/ijms25094826
Gal f-Specific Neolectins: Towards Promising Diagnostic Tools
Abstract
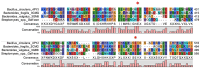
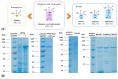
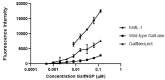
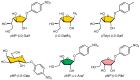
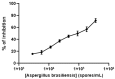
In the absence of naturally available galactofuranose-specific lectin, we report herein the bioengineering of GalfNeoLect, from the first cloned wild-type galactofuranosidase (Streptomyces sp. strain JHA19), which recognises and binds a single monosaccharide that is only related to nonmammalian species, usually pathogenic microorganisms. We kinetically characterised the GalfNeoLect to confirm attenuation of hydrolytic activity and used competitive inhibition assay, with close structural analogues of Galf, to show that it conserved interaction with its original substrate. We synthetised the bovine serum albumin-based neoglycoprotein (GalfNGP), carrying the multivalent Galf units, as a suitable ligand and high-avidity system for the recognition of GalfNeoLect which we successfully tested directly with the galactomannan spores of Aspergillus brasiliensis (ATCC 16404). Altogether, our results indicate that GalfNeoLect has the necessary versatility and plasticity to be used in both research and diagnostic lectin-based applications.
Keywords: galactofuranosidase; neoglycoprotein; neolectin bioengineering; site-directed mutagenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Lindhorst T.K. Essentials of Carbohydrate Chemistry and Biochemistry. 3rd ed. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2007. pp. 5–49.
-
- Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2009. - PubMed
-
- Campo V.L., Aragão-Leoneti V., Teixeira M.B.M., Carvalho I. Carbohydrates and Glycoproteins: Cellular Recognition and Drug Design. In: Taft C.A., editor. New Developments in Medicinal Chemistry. Volume 1. Bentham Science; Sharjah, United Arab Emirates: 2010. pp. 133–151. - DOI
-
- Taylor M.E., Drickamer K. Introduction to Glycobiology. 3rd ed. Oxford University Press; Oxford, UK: 2011. pp. 1–304.
-
- Lichtenthaler F.W. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2010. Carbohydrates: Occurrence, Structures and Chemistry; pp. 15–49. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

