Radiopharmaceuticals for Skeletal Muscle PET Imaging
- PMID: 38732077
- PMCID: PMC11084667
- DOI: 10.3390/ijms25094860
Radiopharmaceuticals for Skeletal Muscle PET Imaging
Abstract
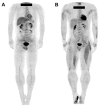
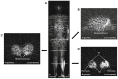
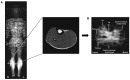
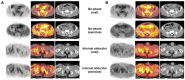
The skeletal muscles account for approximately 40% of the body weight and are crucial in movement, nutrient absorption, and energy metabolism. Muscle loss and decline in function cause a decrease in the quality of life of patients and the elderly, leading to complications that require early diagnosis. Positron emission tomography/computed tomography (PET/CT) offers non-invasive, high-resolution visualization of tissues. It has emerged as a promising alternative to invasive diagnostic methods and is attracting attention as a tool for assessing muscle function and imaging muscle diseases. Effective imaging of muscle function and pathology relies on appropriate radiopharmaceuticals that target key aspects of muscle metabolism, such as glucose uptake, adenosine triphosphate (ATP) production, and the oxidation of fat and carbohydrates. In this review, we describe how [18F]fluoro-2-deoxy-D-glucose ([18F]FDG), [18F]fluorocholine ([18F]FCH), [11C]acetate, and [15O]water ([15O]H2O) are suitable radiopharmaceuticals for diagnostic imaging of skeletal muscles.
Keywords: positron emission tomography; radiopharmaceutical; skeletal muscle atrophy.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design, data collection, analyses, interpretation, writing of the manuscript, or the decision to publish the results.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

