Dihydrotestosterone Augments the Angiogenic and Migratory Potential of Human Endothelial Progenitor Cells by an Androgen Receptor-Dependent Mechanism
- PMID: 38732080
- PMCID: PMC11084206
- DOI: 10.3390/ijms25094862
Dihydrotestosterone Augments the Angiogenic and Migratory Potential of Human Endothelial Progenitor Cells by an Androgen Receptor-Dependent Mechanism
Abstract
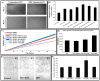
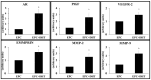
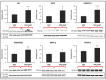
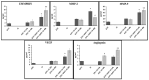
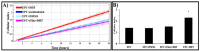
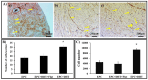
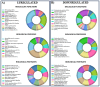
Endothelial progenitor cells (EPCs) play a critical role in cardiovascular regeneration. Enhancement of their native properties would be highly beneficial to ensuring the proper functioning of the cardiovascular system. As androgens have a positive effect on the cardiovascular system, we hypothesized that dihydrotestosterone (DHT) could also influence EPC-mediated repair processes. To evaluate this hypothesis, we investigated the effects of DHT on cultured human EPCs' proliferation, viability, morphology, migration, angiogenesis, gene and protein expression, and ability to integrate into cardiac tissue. The results showed that DHT at different concentrations had no cytotoxic effect on EPCs, significantly enhanced the cell proliferation and viability and induces fast, androgen-receptor-dependent formation of capillary-like structures. DHT treatment of EPCs regulated gene expression of androgen receptors and the genes and proteins involved in cell migration and angiogenesis. Importantly, DHT stimulation promoted EPC migration and the cells' ability to adhere and integrate into murine cardiac slices, suggesting it has a role in promoting tissue regeneration. Mass spectrometry analysis further highlighted the impact of DHT on EPCs' functioning. In conclusion, DHT increases the proliferation, migration, and androgen-receptor-dependent angiogenesis of EPCs; enhances the cells' secretion of key factors involved in angiogenesis; and significantly potentiates cellular integration into heart tissue. The data offer support for potential therapeutic applications of DHT in cardiovascular regeneration and repair processes.
Keywords: angiogenesis; dihydrotestosterone; endothelial progenitor cells; migration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Dihydrotestosterone induces pro-angiogenic factors and assists homing of MSC into the cardiac tissue.J Mol Endocrinol. 2018 Jan;60(1):1-15. doi: 10.1530/JME-17-0185. J Mol Endocrinol. 2018. PMID: 29247133
-
Androgens Stimulate EPC-Mediated Neovascularization and Are Associated with Increased Coronary Collateralization.Endocrinology. 2020 May 1;161(5):bqaa043. doi: 10.1210/endocr/bqaa043. Endocrinology. 2020. PMID: 32157309
-
Androgen Modulates Functions of Endothelial Progenitor Cells through Activated Egr1 Signaling.Stem Cells Int. 2016;2016:7057894. doi: 10.1155/2016/7057894. Epub 2015 Nov 30. Stem Cells Int. 2016. PMID: 26697079 Free PMC article.
-
Androgens and Androgen Receptors as Determinants of Vascular Sex Differences Across the Lifespan.Can J Cardiol. 2022 Dec;38(12):1854-1864. doi: 10.1016/j.cjca.2022.09.018. Epub 2022 Sep 22. Can J Cardiol. 2022. PMID: 36156286 Review.
-
Anti-inflammatory Prowess of endothelial progenitor cells in the realm of biology and medicine.NPJ Regen Med. 2024 Sep 30;9(1):27. doi: 10.1038/s41536-024-00365-z. NPJ Regen Med. 2024. PMID: 39349482 Free PMC article. Review.
References
-
- Asahara T., Murohara T., Sullivan A., Silver M., Van Der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. [(accessed on 1 September 2018)];Science. 1997 275:964–967. doi: 10.1126/science.275.5302.964. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9020076. - DOI - PubMed
-
- Di Stefano R., Felice F., Feriani R., Balbarini A. Endothelial progenitor cells, cardiovascular risk factors and lifestyle modifications. [(accessed on 23 April 2024)];Intern. Emerg. Med. 2013 8:47–49. doi: 10.1007/s11739-013-0915-0. Available online: https://link.springer.com/content/pdf/10.1007%2Fs11739-013-0915-0.pdf. - DOI - PubMed
-
- Vasa M., Fichtlscherer S., Aicher A., Adler K., Urbich C., Martin H., Zeiher A.M., Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. [(accessed on 2 September 2018)];Circ. Res. 2001 89:e1–e7. doi: 10.1161/hh1301.093953. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11440984. - DOI - PubMed
-
- Fadini G.P., Losordo D., Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. [(accessed on 24 September 2018)];Circ. Res. 2012 110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22343557. - DOI - PMC - PubMed
-
- Rosano G.M.C., Spoletini I., Vitale C. Cardiovascular disease in women, is it different to men? The role of sex hormones. [(accessed on 23 April 2024)];Climacteric. 2017 20:125–128. doi: 10.1080/13697137.2017.1291780. Available online: https://www.tandfonline.com/doi/full/10.1080/13697137.2017.1291780. - DOI - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

