Tigecycline Opposes Bortezomib Effect on Myeloma Cells Decreasing Mitochondrial Reactive Oxygen Species Production
- PMID: 38732105
- PMCID: PMC11084384
- DOI: 10.3390/ijms25094887
Tigecycline Opposes Bortezomib Effect on Myeloma Cells Decreasing Mitochondrial Reactive Oxygen Species Production
Abstract
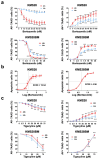
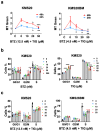
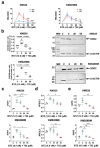
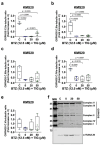
Multiple myeloma is an incurable plasma cell malignancy. Most patients end up relapsing and developing resistance to antineoplastic drugs, like bortezomib. Antibiotic tigecycline has activity against myeloma. This study analyzed tigecycline and bortezomib combination on cell lines and plasma cells from myeloma patients. Apoptosis, autophagic vesicles, mitochondrial mass, mitochondrial superoxide, cell cycle, and hydrogen peroxide were studied by flow cytometry. In addition, mitochondrial antioxidants and electron transport chain complexes were quantified by reverse transcription real-time PCR (RT-qPCR) or western blot. Cell metabolism and mitochondrial activity were characterized by Seahorse and RT-qPCR. We found that the addition of tigecycline to bortezomib reduces apoptosis in proportion to tigecycline concentration. Supporting this, the combination of both drugs counteracts bortezomib in vitro individual effects on the cell cycle, reduces autophagy and mitophagy markers, and reverts bortezomib-induced increase in mitochondrial superoxide. Changes in mitochondrial homeostasis and MYC upregulation may account for some of these findings. These data not only advise to avoid considering tigecycline and bortezomib combination for treating myeloma, but caution on the potential adverse impact of treating infections with this antibiotic in myeloma patients under bortezomib treatment.
Keywords: ROS; antagonism; bortezomib; cell cycle; multiple myeloma; tigecycline.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
-
- Huang J., Chan S.C., Lok V., Zhang L., Lucero-Prisno D.E., 3rd, Xu W., Zheng Z.J., Elcarte E., Withers M., Wong M.C.S. The epidemiological landscape of multiple myeloma: A global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022;9:e670–e677. doi: 10.1016/S2352-3026(22)00165-X. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

