D-Bifunctional Protein Deficiency Diagnosis-A Challenge in Low Resource Settings: Case Report and Review of the Literature
- PMID: 38732138
- PMCID: PMC11084724
- DOI: 10.3390/ijms25094924
D-Bifunctional Protein Deficiency Diagnosis-A Challenge in Low Resource Settings: Case Report and Review of the Literature
Abstract
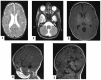
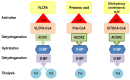
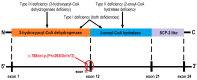
D-bifunctional protein deficiency (D-BPD) is a rare, autosomal recessive peroxisomal disorder that affects the breakdown of long-chain fatty acids. Patients with D-BPD typically present during the neonatal period with hypotonia, seizures, and facial dysmorphism, followed by severe developmental delay and early mortality. While some patients have survived past two years of age, the detectable enzyme activity in these rare cases was likely a contributing factor. We report a D-BPD case and comment on challenges faced in diagnosis based on a narrative literature review. An overview of Romania's first patient diagnosed with D-BPD is provided, including clinical presentation, imaging, biochemical, molecular data, and clinical course. Establishing a diagnosis can be challenging, as the clinical picture is often incomplete or similar to many other conditions. Our patient was diagnosed with type I D-BPD based on whole-exome sequencing (WES) results revealing a pathogenic frameshift variant of the HSD17B4 gene, c788del, p(Pro263GInfs*2), previously identified in another D-BPD patient. WES also identified a variant of the SUOX gene with unclear significance. We advocate for using molecular diagnosis in critically ill newborns and infants to improve care, reduce healthcare costs, and allow for familial counseling.
Keywords: D-bifunctional protein deficiency; neonatal hypotonia; neonatal seizures; peroxisomal disorders; whole-exome sequencing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Dib R.E., Karam P., Mikati M.A., Steinberg S., Habbal M.Z. D-bifunctional protein deficiency, a novel mutation. J. Pediatr. Neurol. 2008;06:357–360. doi: 10.1055/s-0035-1557474. - DOI
-
- Paprocka J., Jamroz E., Adamek D., Stradomska T.J., Głuszkiewicz E., Grzybowska-Chlebowczyk U., Marszał E. Clinical and neuropathological picture of familial encephalopathy with bifunctional protein deficiency. Folia Neuropathol. 2007;45:213–219. - PubMed
-
- McMillan H.J., Worthylake T., Schwartzentruber J., Gottlieb C.C., Lawrence S.E., MacKenzie A., Beaulieu C.L., Mooyer P.A.W., Wanders R.J.A., Majewski J., et al. Specific combination of compound heterozygous mutations in 17β-hydroxysteroid dehydrogenase type 4 (HSD17B4) defines a new subtype of D-bifunctional protein deficiency. Orphanet J. Rare Dis. 2012;7:90. doi: 10.1186/1750-1172-7-90. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

