Exploring Salivary Epithelial Dysfunction in Sjögren's Disease
- PMID: 38732189
- PMCID: PMC11084897
- DOI: 10.3390/ijms25094973
Exploring Salivary Epithelial Dysfunction in Sjögren's Disease
Abstract
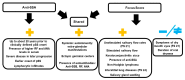
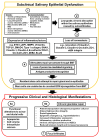
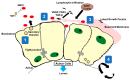
Sjögren's Disease (SjD) is an autoimmune disease of the exocrine tissues. Etiological events result in the loss of epithelial homeostasis alongside extracellular matrix (ECM) destruction within the salivary and lacrimal glands, followed by immune cell infiltration. In this review, we have assessed the current understanding of epithelial-mesenchymal transition (EMT)-associated changes within the salivary epithelium potentially involved in salivary dysfunction and SjD pathogenesis. We performed a PubMed literature review pertaining to the determination of pathogenic events that lead to EMT-related epithelial dysfunction and signaling in SjD. Molecular patterns of epithelial dysfunction in SjD salivary glands share commonalities with EMT mediating wound healing. Pathological changes altering salivary gland integrity and function may precede direct immune involvement while perpetuating MMP9-mediated ECM destruction, inflammatory mediator expression, and eventual immune cell infiltration. Dysregulation of EMT-associated factors is present in the salivary epithelium of SjD and may be significant in initiating and perpetuating the disease. In this review, we further highlight the gap regarding mechanisms that drive epithelial dysfunction in salivary glands in the early or subclinical pre-lymphocytic infiltration stages of SjD.
Keywords: Sjögren’s disease; epithelial–mesenchymal transition; extracellular matrix; salivary gland hypofunction.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
References
-
- Noll B. Ph.D. Thesis. The University of North Carolina at Charlotte; Charlotte, NC, USA: 2022. Dissecting the Salivary Gland: Epithelial-Centered Dysfunction in Primary Sjögren’s Syndrome.
-
- Molina C., Alliende C., Aguilera S., Kwon Y.J., Leyton L., Martínez B., Leyton C., Pérez P., González M.J. Basal lamina disorganisation of the acini and ducts of labial salivary glands from patients with Sjogren’s syndrome: Association with mononuclear cell infiltration. Ann. Rheum. Dis. 2006;65:178–183. doi: 10.1136/ard.2004.033837. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

