Prenatal Choline Supplementation Improves Glucose Tolerance and Reduces Liver Fat Accumulation in Mouse Offspring Exposed to Ethanol during the Prenatal and Postnatal Periods
- PMID: 38732511
- PMCID: PMC11085373
- DOI: 10.3390/nu16091264
Prenatal Choline Supplementation Improves Glucose Tolerance and Reduces Liver Fat Accumulation in Mouse Offspring Exposed to Ethanol during the Prenatal and Postnatal Periods
Abstract
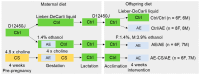
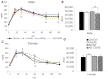
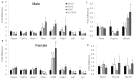
Prenatal alcohol exposure (AE) affects cognitive development. However, it is unclear whether prenatal AE influences the metabolic health of offspring and whether postnatal AE exacerbates metabolic deterioration resulting from prenatal AE. Choline is a semi-essential nutrient that has been demonstrated to mitigate the cognitive impairment of prenatal AE. This study investigated how maternal choline supplementation (CS) may modify the metabolic health of offspring with prenatal and postnatal AE (AE/AE). C57BL/6J female mice were fed either a Lieber-DeCarli diet with 1.4% ethanol between embryonic day (E) 9.5 and E17.5 or a control diet. Choline was supplemented with 4 × concentrations versus the control throughout pregnancy. At postnatal week 7, offspring mice were exposed to 1.4% ethanol for females and 3.9% ethanol for males for 4 weeks. AE/AE increased hepatic triglyceride accumulation in male offspring only, which was normalized by prenatal CS. Prenatal CS also improved glucose tolerance compared to AE/AE animals. AE/AE suppressed hepatic gene expression of peroxisome proliferator activated receptor alpha (Ppara) and low-density lipoprotein receptor (Ldlr), which regulate fatty acid catabolism and cholesterol reuptake, respectively, in male offspring. However, these changes were not rectified by prenatal CS. In conclusion, AE/AE led to an increased risk of steatosis and was partially prevented by prenatal CS in male mice.
Keywords: choline; glucose tolerance; prenatal alcohol exposure; steatosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Iversen M.L., Sørensen N.O., Broberg L., Damm P., Hedegaard M., Tabor A., Hegaard H.K. Alcohol consumption and binge drinking in early pregnancy. A cross-sectional study with data from the Copenhagen Pregnancy Cohort. BMC Pregnancy Childbirth. 2015;15:327. doi: 10.1186/s12884-015-0757-z. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

