TiO2-MoS2-PMMA Nanocomposites for an Efficient Water Remediation
- PMID: 38732669
- PMCID: PMC11085880
- DOI: 10.3390/polym16091200
TiO2-MoS2-PMMA Nanocomposites for an Efficient Water Remediation
Abstract
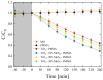
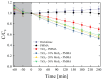
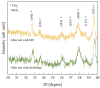
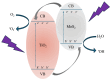
An improvement of water supply and sanitation and better management of water resources, especially in terms of water reuse, is one of the priorities of the European Green Deal. In this context, it is crucial to find new strategies to recycle wastewater efficiently in a low-cost and eco-friendly manner. The immobilization of inorganic nanomaterials on polymeric matrices has been drawing a lot of attention in recent years due to the extraordinary properties characterizing the as-obtained nanocomposites. The hybrid materials, indeed, combine the properties of the polymers, such as flexibility, low cost, mechanical stability, high durability, and ease of availability, with the properties of the inorganic counterpart. In particular, if the inorganic fillers are nanostructured photocatalysts, the materials will be able to utilize the energy delivered by light to catalyze chemical reactions for efficient wastewater treatment. Additionally, with the anchoring of the nanomaterials to the polymers, the dispersion of the nanomaterials in the environment is prevented, thus overcoming one of the main limits that impede the application of nanostructured photocatalysts on a large scale. In this work, we will present nanocomposites made of polymers, i.e., polymethyl methacrylate (PMMA), and photocatalytic semiconductors, i.e., TiO2 nanoparticles (Evonik). MoS2 nanoflakes were also added as co-catalysts to improve the photocatalytic performance of the TiO2. The hybrid materials were prepared using the sonication and solution casting method. The nanocomposites were deeply characterized, and their remarkable photocatalytic abilities were evaluated by the degradation of two common water pollutants: methyl orange and diclofenac. The relevance of the obtained results will be discussed, opening the route for the application of these materials in photocatalysis and especially for novel wastewater remediation.
Keywords: molybdenum disulfide; nanomaterials; photocatalysis; polymer; titanium dioxide; water treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Michalak A.M., Xia J., Brdjanovic D., Mbiyozo A.-N., Sedlak D., Pradeep T., Lall U., Rao N., Gupta J. The Frontiers of Water and Sanitation. Nat. Water. 2023;1:10–18. doi: 10.1038/s44221-022-00020-1. - DOI
-
- Wang J.L., Xu J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012;42:251–325. doi: 10.1080/10643389.2010.507698. - DOI
LinkOut - more resources
Full Text Sources

