Characterization of Chitosan Hydrogels Obtained through Phenol and Tripolyphosphate Anionic Crosslinking
- PMID: 38732743
- PMCID: PMC11085344
- DOI: 10.3390/polym16091274
Characterization of Chitosan Hydrogels Obtained through Phenol and Tripolyphosphate Anionic Crosslinking
Abstract
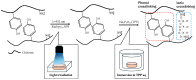
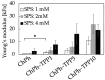
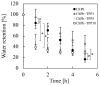
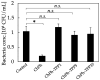
Chitosan is a deacetylated polymer of chitin that is extracted mainly from the exoskeleton of crustaceans and is the second-most abundant polymer in nature. Chitosan hydrogels are preferred for a variety of applications in bio-related fields due to their functional properties, such as antimicrobial activity and wound healing effects; however, the existing hydrogelation methods require toxic reagents and exhibit slow gelation times, which limit their application in biological fields. Therefore, a mild and rapid gelation method is necessary. We previously demonstrated that the visible light-induced gelation of chitosan obtained through phenol crosslinking (ChPh) is a rapid gelation method. To further advance this method (<10 s), we propose a dual-crosslinked chitosan hydrogel obtained by crosslinking phenol groups and crosslinking sodium tripolyphosphate (TPP) and the amino groups of chitosan. The chitosan hydrogel was prepared by immersing the ChPh hydrogel in a TPP solution after phenol crosslinking via exposure to visible light. The physicochemical properties of the dual-crosslinked hydrogels, including Young's moduli and water retentions, were subsequently investigated. Young's moduli of the dual-crosslinked hydrogels were 20 times higher than those of the hydrogels without TPP ion crosslinking. The stiffness could be manipulated by varying the immersion time, and the water retention properties of the ChPh hydrogel were improved by TPP crosslinking. Ion crosslinking could be reversed using an iron chloride solution. This method facilitates chitosan hydrogel use for various applications, particularly tissue engineering and drug delivery.
Keywords: chitosan; hydrogel; phenol group; tripolyphosphate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
An injectable, self-healing phenol-functionalized chitosan hydrogel with fast gelling property and visible light-crosslinking capability for 3D printing.Acta Biomater. 2021 Mar 1;122:211-219. doi: 10.1016/j.actbio.2020.12.051. Epub 2021 Jan 11. Acta Biomater. 2021. PMID: 33444794
-
Autoclaving-Triggered Hydrogelation of Chitosan-Gluconic acid Conjugate Aqueous Solution for Wound Healing.Gels. 2023 Mar 29;9(4):280. doi: 10.3390/gels9040280. Gels. 2023. PMID: 37102892 Free PMC article.
-
A pH-sensitive chitosan-tripolyphosphate hydrogel beads for controlled glipizide delivery.J Biomed Mater Res B Appl Biomater. 2011 Apr;97(1):175-83. doi: 10.1002/jbm.b.31801. Epub 2011 Feb 2. J Biomed Mater Res B Appl Biomater. 2011. PMID: 21290595
-
Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications.J Tissue Eng Regen Med. 2015 Nov;9(11):1225-32. doi: 10.1002/term.1917. Epub 2014 Jun 11. J Tissue Eng Regen Med. 2015. PMID: 24916126 Review.
-
A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing.J Biomater Sci Polym Ed. 2022 Aug;33(12):1595-1622. doi: 10.1080/09205063.2022.2068941. Epub 2022 May 1. J Biomater Sci Polym Ed. 2022. PMID: 35469538 Review.
References
-
- Spicer C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020;11:184–219. doi: 10.1039/c9py01021a. - DOI
-
- Won P., Ko S.H., Majidi C., Feinberg A.W., Webster-Wood V.A. Biohybrid Actuators for Soft Robotics: Challenges in Scaling up. Actuators. 2020;9:96. doi: 10.3390/act9040096. - DOI
LinkOut - more resources
Full Text Sources

