Development of node architecture and emergence of molecular organizer characteristics in the pig embryo
- PMID: 38733144
- PMCID: PMC12344230
- DOI: 10.1002/dvdy.715
Development of node architecture and emergence of molecular organizer characteristics in the pig embryo
Abstract
Background: The avian node is the equivalent of the amphibian Spemann's organizer, as indicated by its ability to induce a secondary axis, cellular contribution, and gene expression, whereas the node of the mouse, which displays limited inductive capacities, was suggested to be a part of spatially distributed signaling. Furthermore, the structural identity of the mouse node is subject of controversy, while little is known about equivalent structures in other mammals.
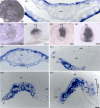
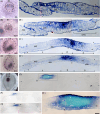
Results: We analyzed the node and emerging organizer in the pig using morphology and the expression of selected organizer genes prior to and during gastrulation. The node was defined according to the "four-quarter model" based on comparative consideration. The node of the pig displays a multilayered, dense structure that includes columnar epithelium, bottle-like cells in the dorsal part, and mesenchymal cells ventrally. Expression of goosecoid (gsc), chordin, and brachyury, together with morphology, reveal the consecutive emergence of three distinct domains: the gastrulation precursor domain, the presumptive node, and the mature node. Additionally, gsc displays a ventral expression domain prior to epiblast epithelialization.
Conclusion: Our study defines the morphological and molecular context of the emerging organizer equivalent in the pig and suggests a sequential development of its function.
Keywords: gastrulation; gene expression; goosecoid; morphogenesis; primitive streak.
© 2024 The Authors. Developmental Dynamics published by Wiley Periodicals LLC on behalf of American Association for Anatomy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

