TWISP: a transgenic worm for interrogating signal propagation in Caenorhabditis elegans
- PMID: 38733622
- PMCID: PMC11228852
- DOI: 10.1093/genetics/iyae077
TWISP: a transgenic worm for interrogating signal propagation in Caenorhabditis elegans
Abstract
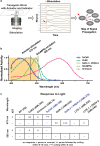
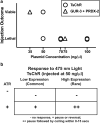
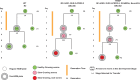
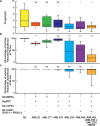
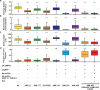
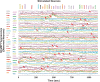
Genetically encoded optical indicators and actuators of neural activity allow for all-optical investigations of signaling in the nervous system. But commonly used indicators, actuators, and expression strategies are poorly suited for systematic measurements of signal propagation at brain scale and cellular resolution. Large-scale measurements of the brain require indicators and actuators with compatible excitation spectra to avoid optical crosstalk. They must be highly expressed in every neuron but at the same time avoid lethality and permit the animal to reach adulthood. Their expression must also be compatible with additional fluorescent labels to locate and identify neurons, such as those in the NeuroPAL cell identification system. We present TWISP, a transgenic worm for interrogating signal propagation, that addresses these needs and enables optical measurements of evoked calcium activity at brain scale and cellular resolution in the nervous system of the nematode Caenorhabditis elegans. In every neuron we express a nonconventional optical actuator, the gustatory receptor homolog GUR-3 + PRDX-2, under the control of a drug-inducible system QF + hGR, and a calcium indicator GCAMP6s, in a background with additional fluorophores from the NeuroPAL cell ID system. We show that this combination, but not others tested, avoids optical crosstalk, creates strong expression in the adult, and generates stable transgenic lines for systematic measurements of signal propagation in the worm brain.
Keywords: Caenorhabditis elegans; GUR-3; PRDX-2; calcium imaging; dexamethasone; drug-inducible gene expression; functional connectivity; neurons; optogenetics.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The author(s) declare no conflicts of interest.
Figures


Update of
-
TWISP: A Transgenic Worm for Interrogating Signal Propagation in C. elegans.bioRxiv [Preprint]. 2023 Aug 5:2023.08.03.551820. doi: 10.1101/2023.08.03.551820. bioRxiv. 2023. Update in: Genetics. 2024 Jul 8;227(3):iyae077. doi: 10.1093/genetics/iyae077. PMID: 37577580 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

