Fractionated radiation therapy alters energy metabolism and induces cellular quiescence exit in patient-derived orthotopic xenograft models of high-grade glioma
- PMID: 38733642
- PMCID: PMC11101904
- DOI: 10.1016/j.tranon.2024.101988
Fractionated radiation therapy alters energy metabolism and induces cellular quiescence exit in patient-derived orthotopic xenograft models of high-grade glioma
Abstract
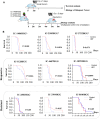
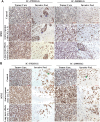
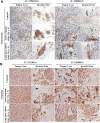
Radiation is one of the standard therapies for pediatric high-grade glioma (pHGG), of which the prognosis remains poor. To gain an in-depth understanding of biological consequences beyond the classic DNA damage, we treated 9 patient-derived orthotopic xenograft (PDOX) models, including one with DNA mismatch repair (MMR) deficiency, with fractionated radiations (2 Gy/day x 5 days). Extension of survival time was noted in 5 PDOX models (P < 0.05) accompanied by γH2AX positivity in >95 % tumor cells in tumor core and >85 % in the invasive foci as well as ∼30 % apoptotic and mitotic catastrophic cell death. The model with DNA MMR (IC-1406HGG) was the most responsive to radiation with a reduction of Ki-67(+) cells. Altered metabolism, including mitochondria number elevation, COX IV activation and reactive oxygen species accumulation, were detected together with the enrichment of CD133+ tumor cells. The latter was caused by the entry of quiescent G0 cells into cell cycle and the activation of self-renewal (SOX2 and BMI1) and epithelial mesenchymal transition (fibronectin) genes. These novel insights about the cellular and molecular mechanisms of fractionated radiation in vivo should support the development of new radio-sensitizing therapies.
Keywords: Cancer stem cells; Glioma; Mitochondrial biogenesis; Orthotopic xenograft; Radiotherapy.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Erker C., Tamrazi B., Poussaint T.Y., Mueller S., Mata-Mbemba D., Franceschi E., et al. Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020;21(6):e317–ee29. - PubMed
-
- Sturm D., Pfister S.M., Jones D.T.W. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J. Clin. Oncol. 2017;35(21):2370–2377. - PubMed
-
- Jakacki R.I., Cohen K.J., Buxton A., Krailo M.D., Burger P.C., Rosenblum M.K., et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children's Oncology Group ACNS0423 study. Neuro Oncol. 2016;18(10):1442–1450. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

