Spinal projecting neurons in rostral ventromedial medulla co-regulate motor and sympathetic tone
- PMID: 38733990
- PMCID: PMC11193620
- DOI: 10.1016/j.cell.2024.04.022
Spinal projecting neurons in rostral ventromedial medulla co-regulate motor and sympathetic tone
Abstract
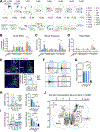
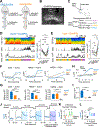
Many behaviors require the coordinated actions of somatic and autonomic functions. However, the underlying mechanisms remain elusive. By opto-stimulating different populations of descending spinal projecting neurons (SPNs) in anesthetized mice, we show that stimulation of excitatory SPNs in the rostral ventromedial medulla (rVMM) resulted in a simultaneous increase in somatomotor and sympathetic activities. Conversely, opto-stimulation of rVMM inhibitory SPNs decreased both activities. Anatomically, these SPNs innervate both sympathetic preganglionic neurons and motor-related regions in the spinal cord. Fiber-photometry recording indicated that the activities of rVMM SPNs correlate with different levels of muscle and sympathetic tone during distinct arousal states. Inhibiting rVMM excitatory SPNs reduced basal muscle and sympathetic tone, impairing locomotion initiation and high-speed performance. In contrast, silencing the inhibitory population abolished muscle atonia and sympathetic hypoactivity during rapid eye movement (REM) sleep. Together, these results identify rVMM SPNs as descending spinal projecting pathways controlling the tone of both the somatomotor and sympathetic systems.
Keywords: blood pressure; medulla; motor control; reticulospinal neurons; spinal projecting neurons; sympathetic regulation; sympathetic tone.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Z.H. is a co-founder of Rugen and Myrobalan and an advisor of Axonis.
Figures





References
-
- Waldrop TG, Eldridge FL, Iwamoto GA, and Mitchell JH (2010). Central neural control of respiration and circulation during exercise. Comprehensive physiology, 333–380.
-
- Michelini LC, O’Leary DS, Raven PB, and Nóbrega AC (2015). Neural control of circulation and exercise: a translational approach disclosing interactions between central command, arterial baroreflex, and muscle metaboreflex. American Journal of Physiology-Heart and Circulatory Physiology 309, H381–H392. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

