Effectiveness of preventive treatment among different age groups and Mycobacterium tuberculosis infection status: a systematic review and individual-participant data meta-analysis of contact tracing studies
- PMID: 38734022
- PMCID: PMC12061052
- DOI: 10.1016/S2213-2600(24)00083-3
Effectiveness of preventive treatment among different age groups and Mycobacterium tuberculosis infection status: a systematic review and individual-participant data meta-analysis of contact tracing studies
Abstract
Background: Tuberculosis is a preventable disease. However, there is debate regarding which individuals would benefit most from tuberculosis preventive treatment and whether these benefits vary in settings with a high burden and low burden of tuberculosis. We aimed to compare the effectiveness of tuberculosis preventive treatment in exposed individuals of differing ages and Mycobacterium tuberculosis infection status while considering tuberculosis burden of the settings.
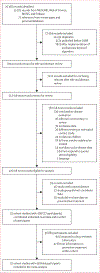
Methods: In this systematic review and individual-participant meta-analysis, we investigated the development of incident tuberculosis in people closely exposed to individuals with tuberculosis. We searched for studies published between Jan 1, 1998, and April 6, 2018, in MEDLINE, Web of Science, BIOSIS, and Embase. We restricted our search to cohort studies; case-control studies and outbreak reports were excluded. Two reviewers evaluated titles, abstracts, and full text articles for eligibility. At each stage, two reviewers discussed discrepancies and re-evaluated articles until a consensus was reached. Individual-participant data and a pre-specified list of variables, including characteristics of the exposed contact, the index patient, and environmental characteristics, were requested from authors of all eligible studies; contacts exposed to a drug-resistant tuberculosis index patient were excluded. The primary study outcome was incident tuberculosis. We estimated adjusted hazard ratios (aHRs) for incident tuberculosis with mixed-effects Cox regression models with a study-level random effect. We estimated the number-needed-to-treat (NNT) to prevent one person developing tuberculosis. Propensity score matching procedures were used in all analyses. This study is registered with PROSPERO (CRD42018087022).
Findings: After screening 25 358 records for eligibility, 439 644 participants from 32 cohort studies were included in the individual-participant data meta-analysis. Participants were followed for 1 396 413 person-years (median of 2·7 years [IQR 1·3-4.4]), during which 2496 people were diagnosed with incident tuberculosis. Overall, effectiveness of preventive treatment was 49% (aHR 0·51 [95% CI 0·44-0·60]). Participants with a positive tuberculin-skin-test (TST) or IFNγ release assay (IGRA) result at baseline benefitted from greater protection, regardless of age (0·09 [0·05-0·17] in children younger than 5 years, 0·20 [0·15-0·28] in individuals aged 5-17 years, and 0·17 [0·13-0·22] in adults aged 18 years and older). The effectiveness of preventive treatment was greater in high-burden (0·31 [0·23-0·40]) versus low-burden (0·58 [0·47-0·72]) settings. The NNT ranged from 9 to 34 depending on age among participants with a positive TST or IGRA in both high-burden and low-burden settings; among all contacts (regardless of TST or IGRA test result), the NNT ranged from 29 to 43 in high-burden settings and 213 to 455 in low-burden settings.
Interpretation: Our findings suggest that a risk-targeted strategy prioritising contacts with evidence of M tuberculosis infection might be indicated in low-burden settings, and a broad approach including all contacts should be considered in high-burden settings. Preventive treatment was similarly effective among contacts of all ages.
Funding: None.
2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures



References
-
- Saunders MJ, Evans CA. Ending tuberculosis through prevention. N Engl J Med 2019; 380: 1073–74. - PubMed
-
- WHO. Global tuberculosis report 2022. Geneva: World Health Organization, 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

