MSC-derived exosomal miR-140-3p improves cognitive dysfunction in sepsis-associated encephalopathy by HMGB1 and S-lactoylglutathione metabolism
- PMID: 38734709
- PMCID: PMC11088640
- DOI: 10.1038/s42003-024-06236-z
MSC-derived exosomal miR-140-3p improves cognitive dysfunction in sepsis-associated encephalopathy by HMGB1 and S-lactoylglutathione metabolism
Abstract
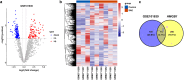
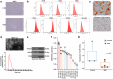
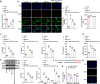
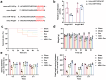
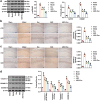
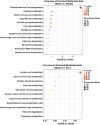
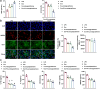
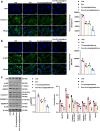
MiRNAs in mesenchymal stem cells (MSCs)-derived exosome (MSCs-exo) play an important role in the treatment of sepsis. We explored the mechanism through which MSCs-exo influences cognitive impairment in sepsis-associated encephalopathy (SAE). Here, we show that miR-140-3p targeted Hmgb1. MSCs-exo plus miR-140-3p mimic (Exo) and antibiotic imipenem/cilastatin (ABX) improve survival, weight, and cognitive impairment in cecal ligation and puncture (CLP) mice. Exo and ABX inhibit high mobility group box 1 (HMGB1), IBA-1, interleukin (IL)-1β, IL-6, iNOS, TNF-α, p65/p-p65, NLRP3, Caspase 1, and GSDMD-N levels. In addition, Exo upregulates S-lactoylglutathione levels in the hippocampus of CLP mice. Our data further demonstrates that Exo and S-lactoylglutathione increase GSH levels in LPS-induced HMC3 cells and decrease LD and GLO2 levels, inhibiting inflammatory responses and pyroptosis. These findings suggest that MSCs-exo-mediated delivery of miR-140-3p ameliorates cognitive impairment in mice with SAE by HMGB1 and S-lactoylglutathione metabolism, providing potential therapeutic targets for the clinical treatment of SAE.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

