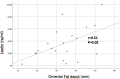
Dapagliflozin added to metformin reduces perirenal fat layer in type 2 diabetic patients with obesity
- PMID: 38734755
- PMCID: PMC11088615
- DOI: 10.1038/s41598-024-61590-6
Dapagliflozin added to metformin reduces perirenal fat layer in type 2 diabetic patients with obesity
Abstract
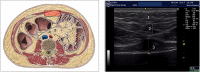
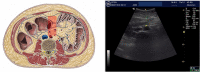
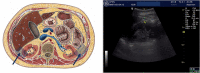
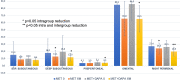
Sodium-glucose co-transporters type 2 inhibitors (SLGT2i) are highly effective in controlling type 2 diabetes, but reported beneficial cardiovascular effects suggest broader actions on insulin resistance. Weight loss may be initially explained by glycosuria-induced net caloric output and secondary volumetric reduction, but its maintenance could be due to loss of visceral fat mass. Structured ultrasound (US) imaging of abdominal adipose tissue ("eco-obesity") is a recently described methodology used to measure 5 consecutive layers of abdominal fat, not assessable by DEXA or CT scan: superficial subcutaneous (SS), deep subcutaneous (DS), preperitoneal (PP), omental (Om) and right perirenal (RK). PP, Om and RK are predictors of metabolic syndrome (MS) with defined cut-off points. To assess the effect of SLGT2i on every fat depot we enrolled 29 patients with type 2 Diabetes (HbA1c 6.5-9%) and Obesity (IMC > 30 kg/m2) in an open-label, randomized, phase IV trial (EudraCT: 2019-000979-16): the Omendapa trial. Diabetes was diagnosed < 12 months before randomization and all patients were treatment naïve. 14 patients were treated with metformin alone (cohort A) and 15 were treated with metformin + dapaglifozin (cohort B). Anthropometric measures and laboratory tests for glucose, lipid profile, insulin, HOMA, leptin, ultrasensitive-CRP and microalbuminuria (MAL) were done at baseline, 3rd and 6th months. At 6th month, weight loss was -5.5 ± 5.2 kg (5.7% from initial weight) in cohort A and -8.4 ± 4.4 kg (8.6%) in cohort B. Abdominal circumference showed a -2.7 ± 3.1 cm and -5.4 ± 2.5 cm reduction, respectively (p = 0.011). Both Metformin alone (-19.4 ± 20.1 mm; -21.7%) or combined with Dapaglifozin (-20.5 ± 19.4 mm; -21.8%) induced significant Om fat reduction. 13.3% of cohort A patients and 21.4% of cohort's B reached Om thickness below the cut-off for MS criteria. RK fat loss was significantly greater in cohort B group compared to cohort A, at both kidneys. Only in the Met + Dapa group, we observed correlations between Om fat with leptin/CRP/MAL and RK fat with HOMA-IR. US is a useful clinical tool to assess ectopic fat depots. Both Metformin and Dapaglifozin induce fat loss in layers involved with MS but combined treatment is particularly effective in perirenal fat layer reduction. Perirenal fat should be considered as a potential target for cardiovascular dapaglifozin beneficial effects.
Keywords: Cardiovascular risk; Dapaglifozin; Metabolic syndrome; Metformin; Omental fat; Perirenal fat; Preperitoneal fat; Ultrasound.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Merovci A, Mari A, Solis-Herrera C, Xiong J, Daniele G, Chavez-Velazquez A, Tripathy D, Urban McCarthy S, Abdul-Ghani M, DeFronzo RA. Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J. Clin. Endocrinol. Metab. 2015;100(5):1927–1932. doi: 10.1210/jc.2014-3472. - DOI - PMC - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM. DAPA-HF trial committees and investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. - DOI - PubMed
-
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. EMPA-REG outcome Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. - DOI - PubMed
-
- Tang H, Fang Z, Wang T, Cui W, Zhai S, Song Y. Meta-analysis of effects of Sodium-glucose cotransporter 2 inhibitors on cardiovascular outcomes and all-cause mortality among patients with type 2 Diabetes mellitus. Am. J. Cardiol. 2016;118(11):1774–1780. doi: 10.1016/j.amjcard.2016.08.061. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous