The MuSK agonist antibody protects the neuromuscular junction and extends the lifespan in C9orf72-ALS mice
- PMID: 38734896
- PMCID: PMC11286808
- DOI: 10.1016/j.ymthe.2024.05.016
The MuSK agonist antibody protects the neuromuscular junction and extends the lifespan in C9orf72-ALS mice
Abstract
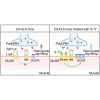
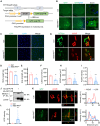
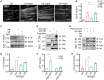
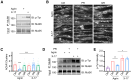
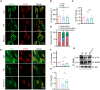
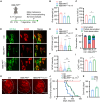
The disassembly of the neuromuscular junction (NMJ) is an early event in amyotrophic lateral sclerosis (ALS), ultimately leading to motor dysfunction and lethal respiratory paralysis. The hexanucleotide GGGGCC repeat expansion in the C9orf72 gene is the most common genetic mutation, and the dipeptide repeat (DPR) proteins have been shown to cause neurodegeneration. While no drugs can treat ALS patients efficiently, new treatment strategies are urgently needed. Here, we report that a MuSK agonist antibody alleviates poly-PR-induced NMJ deficits in C9orf72-ALS mice. The HB9-PRF/F mice, which express poly-PR proteins in motor neurons, exhibited impaired motor behavior and NMJ deficits. Mechanistically, poly-PR proteins interacted with Agrin to disrupt the interaction between Agrin and Lrp4, leading to attenuated activation of MuSK. Treatment with a MuSK agonist antibody rescued NMJ deficits, and extended the lifespan of C9orf72-ALS mice. Moreover, impaired NMJ transmission was observed in C9orf72-ALS patients. These findings identify the mechanism by which poly-PR proteins attenuate MuSK activation and NMJ transmission, highlighting the potential of promoting MuSK activation with an agonist antibody as a therapeutic strategy to protect NMJ function and prolong the lifespan of ALS patients.
Keywords: C9orf72; MuSK; amyotrophic lateral sclerosis; dipeptide repeat proteins; neuromuscular junction.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. - DOI - PMC - PubMed
-
- Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. - DOI - PMC - PubMed
-
- Belzil V.V., Bauer P.O., Prudencio M., Gendron T.F., Stetler C.T., Yan I.K., Pregent L., Daughrity L., Baker M.C., Rademakers R., et al. Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol. 2013;126:895–905. doi: 10.1007/s00401-013-1199-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

