Prophylactic vaccination inducing anti-Env antibodies can result in protection against HTLV-1 challenge in macaques
- PMID: 38734900
- PMCID: PMC11286815
- DOI: 10.1016/j.ymthe.2024.05.020
Prophylactic vaccination inducing anti-Env antibodies can result in protection against HTLV-1 challenge in macaques
Abstract
Human T cell leukemia/T-lymphotropic virus type 1 (HTLV-1) infection occurs by cell-to-cell transmission and can induce fatal adult T cell leukemia. Vaccine development is critical for the control of HTLV-1 transmission. However, determining whether vaccine-induced anti-Env antibodies can prevent cell-to-cell HTLV-1 transmission is challenging. Here, we examined the protective efficacy of a vaccine inducing anti-Env antibodies against HTLV-1 challenge in cynomolgus macaques. Eight of 10 vaccinated macaques produced anti-HTLV-1 neutralizing antibodies (NAbs) and were protected from an intravenous challenge with 108 HTLV-1-producing cells. In contrast, the 2 vaccinated macaques without NAb induction and 10 unvaccinated controls showed HTLV-1 infection with detectable proviral load after challenge. Five of the eight protected macaques were administered with an anti-CD8 monoclonal antibody, but proviruses remained undetectable and no increase in anti-HTLV-1 antibodies was observed even after CD8+ cell depletion in three of them. Analysis of Env-specific T cell responses did not suggest involvement of vaccine-induced Env-specific T cell responses in the protection. These results indicate that anti-Env antibody induction by vaccination can result in functionally sterile HTLV-1 protection, implying the rationale for strategies aimed at anti-Env antibody induction in prophylactic HTLV-1 vaccine development.
Keywords: HTLV-1; cell-to-cell transmission; macaque model; neutralizing antibody; vaccine.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests T.M. is an inventor on Patent Cooperation Treaty (PCT) application for SeV-NVP-EnvF vaccine.
Figures

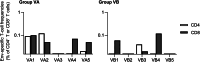




Similar articles
-
Influenza-specific antibody-mediated and complement-dependent cellular cytotoxicity-inducing antibodies in vaccinated and infected pigs.Front Immunol. 2025 Jun 30;16:1600761. doi: 10.3389/fimmu.2025.1600761. eCollection 2025. Front Immunol. 2025. PMID: 40661936 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
An HTLV-1 envelope mRNA vaccine is immunogenic and protective in New Zealand rabbits.J Virol. 2024 Feb 20;98(2):e0162323. doi: 10.1128/jvi.01623-23. Epub 2024 Jan 9. J Virol. 2024. PMID: 38193692 Free PMC article.
-
Virus-host immune interaction in asymptomatic HTLV-1 carriers.Microbiol Spectr. 2025 Mar 4;13(3):e0250724. doi: 10.1128/spectrum.02507-24. Epub 2025 Jan 31. Microbiol Spectr. 2025. PMID: 39887247 Free PMC article.
-
Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors.Cochrane Database Syst Rev. 2018 May 9;5(5):CD009069. doi: 10.1002/14651858.CD009069.pub3. Cochrane Database Syst Rev. 2018. PMID: 29740819 Free PMC article.
Cited by
-
A promising advance in blocking HTLV-1 transmission: Addressing a global public health problem.Mol Ther. 2024 Jul 3;32(7):2050-2051. doi: 10.1016/j.ymthe.2024.06.010. Epub 2024 Jun 21. Mol Ther. 2024. PMID: 38908379 Free PMC article. No abstract available.
-
Human T-Lymphotropic Virus (HTLV): Epidemiology, Genetic, Pathogenesis, and Future Challenges.Viruses. 2025 May 1;17(5):664. doi: 10.3390/v17050664. Viruses. 2025. PMID: 40431676 Free PMC article. Review.
References
-
- Hirons A., Khoury G., Purcell D.F.J. Human T-cell lymphotropic virus type-1: a lifelong persistent infection, yet never truly silent. Lancet Infect. Dis. 2021;21:e2–e10. - PubMed
-
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. - PubMed
-
- Proietti F.A., Carneiro-Proietti A.B.F., Catalan-Soares B.C., Murphy E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

