Cardiovascular Burden of the V142I Transthyretin Variant
- PMID: 38734952
- PMCID: PMC11089467
- DOI: 10.1001/jama.2024.4467
Cardiovascular Burden of the V142I Transthyretin Variant
Abstract
Importance: Individual cohort studies concur that the amyloidogenic V142I variant of the transthyretin (TTR) gene, present in 3% to 4% of US Black individuals, increases heart failure (HF) and mortality risk. Precisely defining carrier risk across relevant clinical outcomes and estimating population burden of disease are important given established and emerging targeted treatments.
Objectives: To better define the natural history of disease in carriers across mid to late life, assess variant modifiers, and estimate cardiovascular burden to the US population.
Design, setting, and participants: A total of 23 338 self-reported Black participants initially free from HF were included in 4 large observational studies across the US (mean [SD], 15.5 [8.2] years of follow-up). Data analysis was performed between May 2023 and February 2024.
Exposure: V142I carrier status (n = 754, 3.2%).
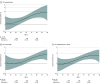
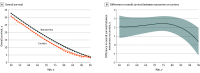
Main outcomes and measures: Hospitalizations for HF (including subtypes of reduced and preserved ejection fraction) and all-cause mortality. Outcomes were analyzed by generating 10-year hazard ratios for each age between 50 and 90 years. Using actuarial methods, mean survival by carrier status was estimated and applied to the 2022 US population using US Census data.
Results: Among the 23 338 participants, the mean (SD) age at baseline was 62 (9) years and 76.7% were women. Ten-year carrier risk increased for HF hospitalization by age 63 years, predominantly driven by HF with reduced ejection fraction, and 10-year all-cause mortality risk increased by age 72 years. Only age (but not sex or other select variables) modified risk with the variant, with estimated reductions in longevity ranging from 1.9 years (95% CI, 0.6-3.1) at age 50 to 2.8 years (95% CI, 2.0-3.6) at age 81. Based on these data, 435 851 estimated US Black carriers between ages 50 and 95 years are projected to cumulatively lose 957 505 years of life (95% CI, 534 475-1 380 535) due to the variant.
Conclusions and relevance: Among self-reported Black individuals, male and female V142I carriers faced similar and substantial risk for HF hospitalization, predominantly with reduced ejection fraction, and death, with steep age-dependent penetrance. Delineating the individual contributions of, and complex interplay among, the V142I variant, ancestry, the social construct of race, and biological or social determinants of health to cardiovascular disease merits further investigation.
Conflict of interest statement
Figures
Comment in
-
Heart Failure in African American Individuals, Version 2.0.JAMA. 2024 Jun 4;331(21):1807-1808. doi: 10.1001/jama.2024.5217. JAMA. 2024. PMID: 38734951 No abstract available.
-
Addressing Health Disparities-The Case for Variant Transthyretin Cardiac Amyloidosis Grows Stronger.JAMA. 2024 Jun 4;331(21):1809-1811. doi: 10.1001/jama.2024.2868. JAMA. 2024. PMID: 38734953 No abstract available.
References
-
- Miller DT, Lee K, Abul-Husn NS, et al. ; ACMG Secondary Findings Working Group . ACMG SF v3.2 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2023;25(8):100866. doi:10.1016/j.gim.2023.100866 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 NS041588/NS/NINDS NIH HHS/United States
- N01 HC095159/HC/NHLBI NIH HHS/United States
- N01 HC095160/HC/NHLBI NIH HHS/United States
- N01 HC095161/HC/NHLBI NIH HHS/United States
- N01 HC095162/HC/NHLBI NIH HHS/United States
- N01 HC095163/HC/NHLBI NIH HHS/United States
- N01 HC095164/HC/NHLBI NIH HHS/United States
- N01 HC095165/HC/NHLBI NIH HHS/United States
- N01 HC095166/HC/NHLBI NIH HHS/United States
- N01 HC095167/HC/NHLBI NIH HHS/United States
- N01 HC095168/HC/NHLBI NIH HHS/United States
- N01 HC095169/HC/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- N02 HL64278/HL/NHLBI NIH HHS/United States
- N02 HL64278/HL/NHLBI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- U01 HG007376/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

