An ovine model for investigation of the microenvironment of the male mammary gland
- PMID: 38735860
- PMCID: PMC11306760
- DOI: 10.1111/joa.14055
An ovine model for investigation of the microenvironment of the male mammary gland
Abstract
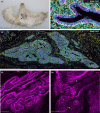
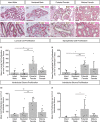
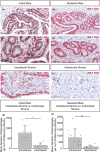
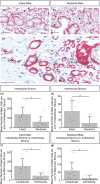
The specific biology of the male breast remains relatively unexplored in spite of the increasing global prevalence of male breast cancer. Delineation of the microenvironment of the male breast is restricted by the low availability of human samples and a lack of characterisation of appropriate animal models. Unlike the mouse, the male ovine gland persists postnatally. We suggest that the male ovine mammary gland constitutes a promising adjunctive model for the male breast. In this study, we evaluate the male ovine mammary gland microenvironment, comparing intact and neutered males. Assessment of the glandular histo-anatomy highlights the resemblance of the male gland to that of neonatal female sheep and confirms the presence of rudimentary terminal duct lobular units. Irrespective of neutered status, cell proliferation in epithelial and stromal compartments is similarly low in males, and cell proliferation in epithelial cells and in the intralobular stroma is significantly lower than in pubertal female sheep. Between 42% and 72% of the luminal mammary epithelial cells in the male gland express the androgen receptor and expression is significantly reduced by neutering. Luminal epithelial cells within the intact and neutered male gland also express oestrogen receptor alpha, but minimal progesterone receptor expression is observed. The distribution of leukocytes within the ducts and stroma is similar to the mammary gland of female sheep and females of other species. Both macrophages and T lymphocytes are intercalated in the epithelial bilayer and are more abundant in the intralobular stroma than the interlobular stroma, suggesting that they may have a protective immunological function within the vestigial glandular tissue of the male sheep. Mast cells are also observed within the stroma. These cells cluster near the glandular tissue and are frequently located adjacent to blood vessels. The abundance of mast cells is significantly higher in intact males compared to neutered males, suggesting that hormone signalling may impact mast cell recruitment. In this study, we demonstrate the utility of the male ovine mammary gland as a model for furthering our knowledge of postnatal male mammary biology.
Keywords: male; mammary gland; microenvironment; model; sheep; udder.
© 2024 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures



References
-
- Cardiff, R.D. , Jindal, S. , Treuting, P.M. , Going, J.J. , Gusterson, B. & Thompson, H.J. (2018) 23. Mammary gland. In: Treuting, P.M., Dintzis, S.M. & Montine, K.S. (Eds.) Comparative anatomy and histology, 2nd edition. San Diego: Academic Press, pp. 487–509. Available from: 10.1016/B978-0-12-802900-8.00023-3 - DOI
-
- Cardoso, F. , Bartlett, J.M.S. , Slaets, L. , van Deurzen, C.H.M. , van Leeuwen‐Stok, E. , Porter, P. et al. (2018) Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Annals of Oncology, Incorporating Blood‐Based Liquid Biopsy Information into Cancer Staging, 29, 405–417. Available from: 10.1093/annonc/mdx651 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

