Human CD4+ iNKT cell adoptive immunotherapy induces anti-tumour responses against CD1d-negative EBV-driven B lymphoma
- PMID: 38736328
- PMCID: PMC11223969
- DOI: 10.1111/imm.13799
Human CD4+ iNKT cell adoptive immunotherapy induces anti-tumour responses against CD1d-negative EBV-driven B lymphoma
Abstract
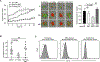
Invariant natural killer T (iNKT) cells are a conserved population of innate T lymphocytes that are uniquely suitable as off-the-shelf cellular immunotherapies due to their lack of alloreactivity. Two major subpopulations of human iNKT cells have been delineated, a CD4- subset that has a TH1/cytolytic profile, and a CD4+ subset that appears polyfunctional and can produce both regulatory and immunostimulatory cytokines. Whether these two subsets differ in anti-tumour effects is not known. Using live cell imaging, we found that CD4- iNKT cells limited growth of CD1d+ Epstein-Barr virus (EBV)-infected B-lymphoblastoid spheroids in vitro, whereas CD4+ iNKT cells showed little or no direct anti-tumour activity. However, the effects of the two subsets were reversed when we tested them as adoptive immunotherapies in vivo using a xenograft model of EBV-driven human B cell lymphoma. We found that EBV-infected B cells down-regulated CD1d in vivo, and administering CD4- iNKT cells had no discernable impact on tumour mass. In contrast, xenotransplanted mice bearing lymphomas showed rapid reduction in tumour mass after administering CD4+ iNKT cells. Immunotherapeutic CD4+ iNKT cells trafficked to both spleen and tumour and were associated with subsequently enhanced responses of xenotransplanted human T cells against EBV. CD4+ iNKT cells also had adjuvant-like effects on monocyte-derived DCs and promoted antigen-dependent responses of human T cells in vitro. These results show that allogeneic CD4+ iNKT cellular immunotherapy leads to marked anti-tumour activity through indirect pathways that do not require tumour cell CD1d expression and that are associated with enhanced activity of antigen-specific T cells.
Keywords: CD1d; EBV; adjuvancy; iNKT cells; lymphoma; tumour immunotherapy.
© 2024 The Authors. Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures


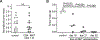

References
-
- Oteo M, Parra JF, Mirones I, Gimenez LI, Setien F, Martinez-Naves E. Single strand conformational polymorphism analysis of human CD1 genes in different ethnic groups. Tissue Antigens 1999; 53:545–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

