Ethylene biosynthesis and signal transduction during ripening and softening in non-climacteric fruits: an overview
- PMID: 38736445
- PMCID: PMC11082881
- DOI: 10.3389/fpls.2024.1368692
Ethylene biosynthesis and signal transduction during ripening and softening in non-climacteric fruits: an overview
Abstract
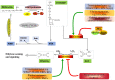
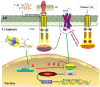
In recent years, the ethylene-mediated ripening and softening of non-climacteric fruits have been widely mentioned. In this paper, recent research into the ethylene-mediated ripening and softening of non-climacteric fruits is summarized, including the involvement of ethylene biosynthesis and signal transduction. In addition, detailed studies on how ethylene interacts with other hormones to regulate the ripening and softening of non-climacteric fruits are also reviewed. These findings reveal that many regulators of ethylene biosynthesis and signal transduction are linked with the ripening and softening of non-climacteric fruits. Meanwhile, the perspectives of future research on the regulation of ethylene in non-climacteric fruit are also proposed. The overview of the progress of ethylene on the ripening and softening of non-climacteric fruit will aid in the identification and characterization of key genes associated with ethylene perception and signal transduction during non-climacteric fruit ripening and softening.
Keywords: ethylene biosynthesis; ethylene signal transduction; non-climacteric fruit; ripening; softening.
Copyright © 2024 Liu, Wang, Ji, Sun, Liu, Wang, Cao and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Different regulatory mechanisms of plant hormones in the ripening of climacteric and non-climacteric fruits: a review.Plant Mol Biol. 2021 Dec;107(6):477-497. doi: 10.1007/s11103-021-01199-9. Epub 2021 Oct 11. Plant Mol Biol. 2021. PMID: 34633626 Review.
-
The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene-An overview.J Food Sci Technol. 2012 Feb;49(1):1-21. doi: 10.1007/s13197-011-0293-4. Epub 2011 Feb 11. J Food Sci Technol. 2012. PMID: 23572821 Free PMC article.
-
A non-climacteric fruit gene CaMADS-RIN regulates fruit ripening and ethylene biosynthesis in climacteric fruit.PLoS One. 2014 Apr 21;9(4):e95559. doi: 10.1371/journal.pone.0095559. eCollection 2014. PLoS One. 2014. PMID: 24751940 Free PMC article.
-
Atypical Climacteric and Functional Ethylene Metabolism and Signaling During Fruit Ripening in Blueberry (Vaccinium sp.).Front Plant Sci. 2022 Jun 23;13:932642. doi: 10.3389/fpls.2022.932642. eCollection 2022. Front Plant Sci. 2022. PMID: 35812961 Free PMC article.
-
Ethylene receptors and related proteins in climacteric and non-climacteric fruits.Plant Sci. 2018 Nov;276:63-72. doi: 10.1016/j.plantsci.2018.07.012. Epub 2018 Aug 10. Plant Sci. 2018. PMID: 30348329 Review.
Cited by
-
Genome-wide identification and expression analysis of ACS and ACO gene family in Ziziphus jujuba mill during fruit ripening.Sci Rep. 2025 May 24;15(1):18106. doi: 10.1038/s41598-025-03014-7. Sci Rep. 2025. PMID: 40413254 Free PMC article.
-
EIL (ethylene-insensitive 3-like) transcription factors in apple affect both ethylene- and cold response-dependent fruit ripening.Plant J. 2025 Mar;121(5):e70059. doi: 10.1111/tpj.70059. Plant J. 2025. PMID: 40051338 Free PMC article.
-
Blueberry ripening mechanism: a systematic review of physiological and molecular evidence.Hortic Res. 2025 May 14;12(8):uhaf126. doi: 10.1093/hr/uhaf126. eCollection 2025 Aug. Hortic Res. 2025. PMID: 40666674 Free PMC article.
References
-
- Alós E., Martinez-Fuentes A., Reig C., Mesejo C., Zacarías L., Agustí M., et al. . (2019). Involvement of ethylene in color changes and carotenoid biosynthesis in loquat fruit (Eriobotrya japonica Lindl. cv. Algerie). Postharvest Biol. Technol. 149, 129–138. doi: 10.1016/j.postharvbio.2018.11.022 - DOI
Publication types
LinkOut - more resources
Full Text Sources

