A vascularized in vivo melanoma model suitable for metastasis research of different tumor stages using fundamentally different bioinks
- PMID: 38736612
- PMCID: PMC11081803
- DOI: 10.1016/j.mtbio.2024.101071
A vascularized in vivo melanoma model suitable for metastasis research of different tumor stages using fundamentally different bioinks
Abstract
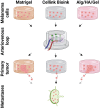
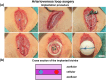
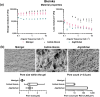
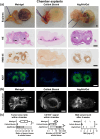
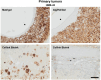
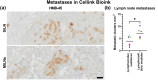
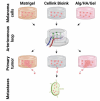
Although 2D cancer models have been the standard for drug development, they don't resemble in vivo properties adequately. 3D models can potentially overcome this. Bioprinting is a promising technique for more refined models to investigate central processes in tumor development such as proliferation, dormancy or metastasis. We aimed to analyze bioinks, which could mimic these different tumor stages in a cast vascularized arteriovenous loop melanoma model in vivo. It has the advantage to be a closed system with a defined microenvironment, supplied only with one vessel-ideal for metastasis research. Tested bioinks showed significant differences in composition, printability, stiffness and microscopic pore structure, which led to different tumor stages (Matrigel and Alg/HA/Gel for progression, Cellink Bioink for dormancy) and resulted in different primary tumor growth (Matrigel significantly higher than Cellink Bioink). Light-sheet fluorescence microscopy revealed differences in vascularization and hemorrhages with no additional vessels found in Cellink Bioink. Histologically, typical human melanoma with different stages was demonstrated. HMB-45-positive tumors in progression inks were infiltrated by macrophages (CD163), highly proliferative (Ki67) and metastatic (MITF/BRN2, ATX, MMP3). Stainings of lymph nodes revealed metastases even without significant primary tumor growth in Cellink Bioink. This model can be used to study tumor pathology and metastasis of different tumor stages and therapies.
Keywords: Bioink; Melanoma; Metastasis; Tissue engineering; Tumor.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts.Tissue Eng Part A. 2021 Sep;27(17-18):1168-1181. doi: 10.1089/ten.TEA.2020.0305. Epub 2021 Feb 26. Tissue Eng Part A. 2021. PMID: 33218292 Free PMC article.
-
Comparison of the Behavior of 3D-Printed Endothelial Cells in Different Bioinks.Bioengineering (Basel). 2023 Jun 23;10(7):751. doi: 10.3390/bioengineering10070751. Bioengineering (Basel). 2023. PMID: 37508778 Free PMC article.
-
Peptide-dendrimer-reinforced bioinks for 3D bioprinting of heterogeneous and biomimetic in vitro models.Acta Biomater. 2023 Oct 1;169:243-255. doi: 10.1016/j.actbio.2023.08.008. Epub 2023 Aug 11. Acta Biomater. 2023. PMID: 37572980
-
Strategies for improving the 3D printability of decellularized extracellular matrix bioink.Theranostics. 2023 Apr 23;13(8):2562-2587. doi: 10.7150/thno.81785. eCollection 2023. Theranostics. 2023. PMID: 37215563 Free PMC article. Review.
-
Hyaluronic Acid as Bioink and Hydrogel Scaffolds for Tissue Engineering Applications.ACS Biomater Sci Eng. 2023 Jun 12;9(6):3134-3159. doi: 10.1021/acsbiomaterials.3c00299. Epub 2023 Apr 28. ACS Biomater Sci Eng. 2023. PMID: 37115515 Review.
Cited by
-
Modeling a mesenchymal cell state by bioprinting for the molecular analysis of dormancy in melanoma.Mater Today Bio. 2025 Mar 18;32:101674. doi: 10.1016/j.mtbio.2025.101674. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40206148 Free PMC article.
-
Plasticity of Expression of Stem Cell and EMT Markers in Breast Cancer Cells in 2D and 3D Culture Depend on the Spatial Parameters of Cell Growth; Mathematical Modeling of Mechanical Stress in Cell Culture in Relation to ECM Stiffness.Bioengineering (Basel). 2025 Feb 4;12(2):147. doi: 10.3390/bioengineering12020147. Bioengineering (Basel). 2025. PMID: 40001667 Free PMC article.
-
Characterization of two different alginate-based bioinks and the influence of melanoma growth within.Sci Rep. 2024 Jun 5;14(1):12945. doi: 10.1038/s41598-024-63642-3. Sci Rep. 2024. PMID: 38839791 Free PMC article.
References
-
- Yao B., et al. Enzymatically degradable alginate/gelatin bioink promotes cellular behavior and degradation in vitro and in vivo. Biofabrication. 2019;11(4) - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

