The nature of chronic rejection after lung transplantation: a murine orthotopic lung transplant study
- PMID: 38736881
- PMCID: PMC11084670
- DOI: 10.3389/fimmu.2024.1369536
The nature of chronic rejection after lung transplantation: a murine orthotopic lung transplant study
Abstract
Introduction: Chronic rejection is a major complication post-transplantation. Within lung transplantation, chronic rejection was considered as airway centred. Chronic Lung Allograft Dysfunction (CLAD), defined to cover all late chronic complications, makes it more difficult to understand chronic rejection from an immunological perspective. This study investigated the true nature, timing and location of chronic rejection as a whole, within mouse lung transplantation.
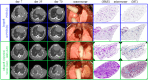
Methods: 40 mice underwent an orthotopic left lung transplantation, were sacrificed at day 70 and evaluated by histology and in vivo µCT. For timing and location of rejection, extra grafts were sacrificed at day 7, 35, 56 and investigated by ex vivo µCT or single cell RNA (scRNA) profiling.
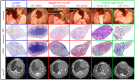
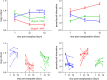
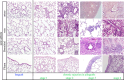
Results: Chronic rejection originated as innate inflammation around small arteries evolving toward adaptive organization with subsequent end-arterial fibrosis and obliterans. Subsequently, venous and pleural infiltration appeared, followed by airway related bronchiolar folding and rarely bronchiolitis obliterans was observed. Ex vivo µCT and scRNA profiling validated the time, location and sequence of events with endothelial destruction and activation as primary onset.
Conclusion: Against the current belief, chronic rejection in lung transplantation may start as an arterial response, followed by responses in venules, pleura, and, only in the late stage, bronchioles, as may be seen in some but not all patients with CLAD.
Keywords: chronic rejection; imaging; lung transplantation; mouse model; single-cell profiling.
Copyright © 2024 Heigl, Kaes, Aelbrecht, Serré, Yamada, Geudens, Van Herck, Vanstapel, Sacreas, Ordies, Frick, Saez Gimenez, Van Slambrouck, Beeckmans, Acet Oztürk, Orlitova, Vaneylen, Claes, Schols, Vande Velde, Schupp, Kaminski, Boesch, Korf, van der Merwe, Dupont, Vanoirbeek, Godinas, Van Raemdonck, Janssens, Gayan-Ramirez, Ceulemans, McDonough, Verbeken, Vos and Vanaudenaerde.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Li XC, Jevnikar AM. Transplant immunology. Houston, USA: Wiley-Blackwell; (2015). doi: 10.1002/9781119072997 - DOI
-
- Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, et al. . Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. (2019) 38:493–503. doi: 10.1016/j.healun.2019.03.009 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

