Once-weekly semaglutide versus placebo in adults with increased fracture risk: a randomised, double-blinded, two-centre, phase 2 trial
- PMID: 38737002
- PMCID: PMC11087719
- DOI: 10.1016/j.eclinm.2024.102624
Once-weekly semaglutide versus placebo in adults with increased fracture risk: a randomised, double-blinded, two-centre, phase 2 trial
Abstract
Background: Previous studies have indicated that glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) may enhance bone formation and have neutral or beneficial effects on fracture risk. We evaluated the effect of the GLP-1RA semaglutide on the bone formation marker Procollagen type I N-terminal propeptide (PINP) in adults with increased fracture risk.
Methods: This randomised, placebo-controlled, double-blinded, phase 2 clinical trial was conducted at two public hospitals in Denmark. We enrolled 64 men and women with increased fracture risk based on a T-score < -1.0 at the total hip or lumbar spine and/or low-energy fracture within three years of recruitment. Participants were randomised (1:1) to receive once-weekly subcutaneous semaglutide 1.0 mg or placebo. The primary outcome was changes in plasma (P)-PINP from baseline to week 52. Primary and safety outcomes were assessed and evaluated for all participants. This trial is complete and registered with ClinicalTrials.gov, NCT04702516.
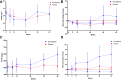
Findings: Between March 24 and December 8, 2021, 55 (86%) postmenopausal women and nine men with a mean age of 63 years (SD 5.5) and BMI of 27.5 kg/m2 (SD 4.5) were enrolled. There was no effect on changes in P-PINP from baseline to week 52 between the two groups (estimated treatment difference (ETD) semaglutide versus placebo 3.8 μg/L [95% CI -5.6 to 13.3]; p = 0.418), and no difference in P-PINP levels between groups at week 52 (semaglutide 64.3 μg/L versus placebo 62.3 μg/L [95% CI -10.8 to 15.0]; p = 0.749). The secondary outcomes showed higher plasma levels of bone resorption marker Collagen type I cross-linked C-terminal telopeptide (P-CTX) in the semaglutide group than in the placebo group (ETD 166.4 ng/L [95% CI 25.5-307.3]; p = 0.021). Compared to placebo, lumbar spine and total hip areal bone mineral densities (aBMD) were lower in the semaglutide group after 52 weeks ((ETD lumbar spine -0.018 g/cm3 [95% CI -0.031 to -0.005]; p = 0.007); ETD total hip -0.020 g/cm2 ([95% CI -0.032 to -0.008]; p = 0.001). Treatment differences in femoral neck aBMD were not observed ([95% CI [-0.017 to 0.006]; p = 0.328). Further, body weight was lower in the semaglutide group than in the placebo group after 52 weeks (ETD -6.8 kg [95% CI -8.8 to -4.7]; p < 0.001). Thirty-one [97%] in the semaglutide group and 18 [56%] in the placebo group experienced at least one adverse event, including four serious events (two in each group). No episodes of hypoglycaemia or deaths were reported.
Interpretation: In adults with increased fracture risk, semaglutide once weekly did not increase bone formation based on the bone formation marker P-PINP. The observed increase in bone resorption in the semaglutide group may be explained by the accompanying weight loss.
Funding: Region of Southern Denmark, Novo Nordisk Foundation, and Gangsted Foundation. Novo Nordisk provided the investigational drug and placebo.
Keywords: Bone turnover; Cortical bone; Semaglutide; Weight loss.
© 2024 The Author(s).
Conflict of interest statement
EMW, SJ, SGH, JJM, and CE declare no conflicts of interest. MSH and MF have received funding from the Novo Nordisk Foundation. RE receives consultancy funding from Immunodiagnostic Systems, Sandoz, Samsung, CL Bio, Biocon, Takeda, UCB, meeting presentations for Pharmacosmos, Alexion, UCB and Amgen, and grant funding from Alexion. NRJ has received assays and reagents from IDS and Roche for clinical studies. MF has received consultancy funding from Novo Nordisk and is shareholder at Novo Nordisk and Eli Lily. Novo Nordisk, Denmark, provided the investigational drug and placebo.
Figures
References
-
- Szulc P., Seeman E. Thinking inside and outside the envelopes of bone: dedicated to PDD. Osteoporos Int. 2009;20:1281–1288. - PubMed
-
- Ensrud K.E. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013;68:1236–1242. - PubMed
-
- Shoback D., Rosen C.J., Black D.M., Cheung A.M., Murad M.H., Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society guideline update. J Clin Endocrinol Metab. 2020;105:1595–1622. - PubMed
-
- Cipriani C., Pepe J., Minisola S., Lewiecki E.M. Adverse effects of media reports on the treatment of osteoporosis. J Endocrinol Invest. 2018;41:1359–1364. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

